Dendrite
| Dendrite |
|---|
Dendrites (from Greek δένδρον déndron, “tree”) are the branched projections of a neuron that act to conduct the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic arbor. Dendrites play a critical role in integrating these synaptic inputs and in determining the extent to which action potentials are produced by the neuron. Recent research has also found that dendrites can support action potentials and release neurotransmitters, a property that was originally believed to be specific to axons.
The long outgrowths on dendritic cells are also called dendrites. These dendrites do not process electrical signals.
Contents |
Electrical properties of dendrites
The structure and branching of a neuron's dendrites, as well as the availability and variation in voltage-gated ion conductances, strongly influences how it integrates the input from other neurons, particularly those that input only weakly. This integration is both "temporal" -- involving the summation of stimuli that arrive in rapid succession -- as well as "spatial" -- entailing the aggregation of excitatory and inhibitory inputs from separate branches.
Dendrites were once believed to merely convey stimulation passively. In this example, voltage changes measured at the cell body result from activations of distal synapses propagating to the soma without the aid of voltage-gated ion channels. Passive cable theory describes how voltage changes at a particular location on a dendrite transmit this electrical signal through a system of converging dendrite segments of different diameters, lengths, and electrical properties. Based on passive cable theory one can track how changes in a neuron’s dendritic morphology changes the membrane voltage at the soma, and thus how variation in dendrite architectures affects the overall output characteristics of the neuron.
Although passive cable theory offers insights regarding input propagation along dendrite segments, it is important to remember that dendrite membranes are host to a cornucopia of proteins some of which may help amplify or attenuate synaptic input. Sodium, calcium, and potassium channels are all implicated in contributing to input modulation. It is possible that each of these ion species has a family of channel types each with its own biophysical characteristics relevant to synaptic input modulation. Such characteristics include the latency of channel opening, the electrical conductance of the ion pore, the activation voltage, and the activation duration. In this way, a weak input from a distal synapse can be amplified by sodium and calcium currents en route to the soma so that the effects of distal synapse are no less robust than those of a proximal synapse.
One important feature of dendrites, endowed by their active voltage gated conductances, is their ability to send action potentials back into the dendritic arbor. Known as backpropagating action potentials, these signals depolarize the dendritic arbor and provide a crucial component toward synapse modulation and long-term potentiation.Furthermore, a train of backpropagating action potentials artificially generated at the soma can induce a calcium action potential at the dendritic initiation zone in certain types of neurons. Whether or not this mechanism is of physiological importance remains an open question.
Dendrite development
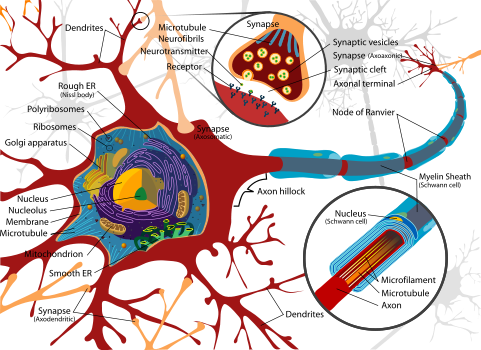
Despite the critical role that dendrites play in the computational tendencies of neurons, very little is known about the process by which dendrites orient themselves in vivo and are compelled to create the intricate branching pattern unique to each specific neuronal class. It is likely that a complex array of extracellular and intracellular cues modulate dendrite development. Early candidates include: Sema3A, Notch, CREST, and Dasm1. Sema3A may act as a dendritic chemoattractant that aids cortical pyramidal neurons in orienting their apical dendrites to the pial surface. Notch acts as a neurotrophic factor in aiding dendrite growth and branching, while CREST may play an important role in regulating calcium dependent growth signals. Dasm1 (Dendrite arborization and synapse maturation 1) expression appears to be highly localized to dendrites and may have substantial influence on dendrite (but not axon) development.
See also
- Dendritic spine
- Axon
- Synapse
- Purkinje cell
- Pyramidal neuron
References
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science, 4th ed. McGraw-Hill, New York (2000). ISBN 0-8385-7701-6
- Koch C. Biophysics of Computation, Oxford University Press, Oxford (1999). ISBN 0-19-510491-9
- Stuart G, Spruston N, Hausser M. Dendrites, Oxford University Press, USA (2008). ISBN 0-1985-6656-5
External links
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
