Darmstadtium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| unknown | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name, symbol, number | darmstadtium, Ds, 110 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | darm-SHTAT-ee-əm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 10, 7, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [281]g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn]7s15f146d9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 17, 1 (Image) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | unknown | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 54083-77-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main article: Isotopes of darmstadtium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Darmstadtium (pronounced /dɑrmˈʃtætiəm/ (![]() listen) darm-SHTAT-ee-əm), formerly known as ununnilium, is a chemical element with the symbol Ds and atomic number 110. It is placed as the heaviest member of group 10 but a sufficiently stable isotope is not known which would allow chemical experiments to confirm its place. This synthetic element is one of the so-called super-heavy atoms and was first synthesized in 1994. The longest-lived and heaviest isotope known is 281aDs with a half-life of ~10 s although a possible nuclear isomer, 281bDs has an unconfirmed half-life of about 4 minutes.
listen) darm-SHTAT-ee-əm), formerly known as ununnilium, is a chemical element with the symbol Ds and atomic number 110. It is placed as the heaviest member of group 10 but a sufficiently stable isotope is not known which would allow chemical experiments to confirm its place. This synthetic element is one of the so-called super-heavy atoms and was first synthesized in 1994. The longest-lived and heaviest isotope known is 281aDs with a half-life of ~10 s although a possible nuclear isomer, 281bDs has an unconfirmed half-life of about 4 minutes.
History
Official discovery
Darmstadtium was first created on November 9, 1994 at the Gesellschaft für Schwerionenforschung (GSI) in Wixhausen, a northern suburb of Darmstadt, Germany by Peter Armbruster and Gottfried Münzenberg, under the direction of professor Sigurd Hofmann. Four atoms of it were detected by a nuclear fusion reaction caused by bombarding a lead-208 target with nickel-62 ions: [1]
- 20882Pb + 6228Ni → 269110Ds + 10n
In the same series of experiments, the same team also carried out the reaction using heavier nickel-64 ions. During two runs, 9 atoms of 271Ds were convincingly detected by correlation with known daughter decay properties: [2]
- 20882Pb + 6428Ni → 271110Ds + 10n
The IUPAC/IUPAP Joint Working Party (JWP) recognised the GSI team as discoverers in their 2001 report.[3]
Proposed names
Element 110 was first given the temporary name ununnilium (/ˌjuːnəˈnɪliəm/ or /ˌʌnəˈnɪliəm/[4], symbol Uun). Once recognized as discoverers, the team at GSI considered the names darmstadtium (Ds) and wixhausium (Wi) for element 110. They decided on the former and named the element after the city near the place of its discovery, Darmstadt and not the suburb Wixhausen itself. The new name was officially recommended by IUPAC on August 16, 2003.
Future experiments
The team at GSI has scheduled experiments for Aug 27 - Oct 10 2010 in order to re-study the K-isomer of 270Ds formed in the reaction 207Pb(64Ni,n).
The team at the HIRFL, Lanzhou, China are planning to restudy the reaction 238U(40Ar,xn) after recent calculations indicated a measurable yield in the 4n evaporation channel, leading to the new nuclide 274Ds.
At the FLNR, scientists will study the new reaction 226Ra(50Ti,xn) in order to compare the yield with that obtained using 48Ca projectiles in order to ascertain the viability of using 50Ti projectiles in SHE synthesis.
Isotopes and nuclear properties
Nucleosynthesis
Target-Projectile Combinations leading to Z=110 compound nuclei
The below table contains various combinations of targets and projectiles which could be used to form compound nuclei with Z=110.
| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 208Pb | 64Ni | 272Ds | Successful reaction |
| 208Pb | 62Ni | 270Ds | Successful reaction |
| 232Th | 48Ca | 280Ds | Failure to date |
| 238U | 40Ar | 278Ds | Failure to date |
| 244Pu | 36S | 280Ds | Reaction yet to be attempted |
| 244Pu | 34S | 278Ds | Successful reaction |
| 248Cm | 30Si | 278Ds | Reaction yet to be attempted |
| 249Cf | 26Mg | 275Ds | Reaction yet to be attempted |
Cold fusion
This section deals with the synthesis of nuclei of darmstadtium by so-called "cold" fusion reactions. These are processes which create compound nuclei at low excitation energy (~10-20 MeV, hence "cold"), leading to a higher probability of survival from fission. The excited nucleus then decays to the ground state via the emission of one or two neutrons only.
208Pb(64Ni,xn)272-xDs (x=1)
This reaction was first studied by scientists at GSI in 1986, without success. A cross section limit of 12 pb was calculated. After an upgrade of their facilities, they successfully detected 9 atoms of 271Ds in two runs in 1994 as part of their discovery experiments on element 110.[2] This reaction was successfully repeated in 2000 by GSI (4 atoms), in 2000 [5] [6] and 2004 [7] [8] by LBNL (9 atoms in total) and in 2002 by RIKEN (14 atoms).[9] The summation of the data allowed a measurement of the 1n neutron evaporation excitation function.
207Pb(64Ni,xn)271-xDs (x=1)
In addition to the official discovery reactions, in October-November 2000, the team at GSI also studied the reaction using a Pb-207 target in order to search for the new isotope 270Ds. They succeeded in synthesising 8 atoms of 270Ds, relating to a ground state isomer, 270gDs, and a high-spin K-isomer, 270mDs. [10]
208Pb(62Ni,xn)270-xDs (x=1)
The GSI team studied this reaction in 1994 as part of their discovery experiment. Three atoms of 269Ds were detected.[1] A fourth decay chain was measured but subsequently retracted.
209Bi(59Co,xn)268-xDs
This reaction was first studied by the team at Dubna in 1986. They were unable to detect any product atoms and measured a cross section limit of 1 pb. In 1995, the team at LBNL reported that they had succeeded in detecting a single atom of 267Ds from the 1n neutron evaporation channel. However, several decays were missed and further research is required to confirm this discovery. [11]
Hot fusion
This section deals with the synthesis of nuclei of darmstadtium by so-called "hot" fusion reactions. These are processes which create compound nuclei at high excitation energy (~40-50 MeV, hence "hot"), leading to a reduced probability of survival from fission. The excited nucleus then decays to the ground state via the emission of 3-5 neutrons. Fusion reactions utilizing 48Ca nuclei usually produce compound nuclei with intermediate excitation energies (~30-35 MeV) and are sometimes referred to as "warm" fusion reactions. This leads, in part, to relatively high yields from these reactions.
232Th(48Ca,xn)280-xDs
The synthesis of element 110 by hot fusion pathways was first attempted in 1986 by the team at Dubna. Using the method of detection of spontaneous fission, they were unable to measure any SF activities and calculated a cross section limit of 1 pb for the decay mode. In three separate experiments between November 1997 and October 1998, the same team re-studied this reaction as part of their new 48Ca program on the synthesis of superheavy elements. Several SF activities with relatively long half-lives were detected and tentatively assigned to decay of the daughters 269Sg or 265Rf, with a cross section of 5 pb. These observations have not been confirmed and the results are taken as only an indication for the synthesis of darmstadtium in this reaction.
232Th(44Ca,xn)276-xDs
This reaction was attempted in 1986 and 1987 by the Dubna team. In both experiments, a 10 ms SF activities was measured and assigned to 272Ds, with a calculated cross section of 10 pb. This activity is currently not thought to be due to a darmstadtium isotope.
238U(40Ar,xn)278-xDs
This reaction was first attempted by the Dubna team in 1987. Only spontaneous fission from the transfer products 240mfAm and 242mfAm were observed and the team calculated a cross section limit of 1.6 pb. The team at GSI first studied this reaction in 1990. Once again, no atoms of element 110 could be detected. In August 2001, the GSI repeated reaction, without success, and calculated a cross section limit of 1.0 pb.
236U(40Ar,xn)276-xDs
This reaction was first attempted by the Dubna team in 1987. No spontaneous fission was observed.
235U(40Ar,xn)275-xDs
This reaction was first attempted by the Dubna team in 1987. No spontaneous fission was observed. It was further studied in 1990 by the GSI team. Once again, no atoms were detected and a cross section limit of 21 pb was calculated.
233U(40Ar,xn)273-xDs
This reaction was first studied in 1990 by the GSI team. No atoms were detected and a cross section limit of 21 pb was calculated.
244Pu(34S,xn)278-xDs (x=5)
In September 1994 the team at Dubna detected a single atom of 273Ds, formed in the 5n neutron evaporation channel. The measured cross section was just 400 fb. [12]
As a decay product
Isotopes of darmstadtium have also been detected in the decay of heavier elements. Observations to date are shown in the table below:
| Evaporation Residue | Observed Ds isotope |
|---|---|
| 293Uuh, 289Uuq | 281Ds |
| 291Uuh, 287Uuq, 283Cn | 279Ds |
| 277Cn | 273Ds |
In some experiments, the decay of 293Uuh and 289Uuq produced an isotope of darmstadtium decaying by emission of an 8.77 MeV alpha particle with a half life of 3.7 minutes. Although unconfirmed, it is highly possible that this activity is associated with a meta-stable isomer, namely 281mDs.
Retracted isotopes
280Ds
The first synthesis of element 114 resulted in two atoms assigned to 288Uuq, decaying to the 280Ds which underwent spontaneous fission. The assignment was later changed to 289Uuq and the darmstadtium isotope to 281Ds. Hence, 280Ds is currently unknown.
277Ds
In the claimed synthesis of 293Uuo in 1999, the isotope 277Ds was identified as decaying by 10.18 MeV alpha emission with a half-life of 3.0 ms. This claim was retracted in 2001 and thus this darmstadtium isotope is currently unknown or unconfirmed.[13]
273mDs
In the synthesis of 277Cn in 1996 by GSI (see copernicium), one decay chain proceeded via 273Ds which decayed by emission of a 9.73 MeV alpha particle with a lifetime of 170 ms. This would have been assigned to an isomeric level. This data could not be confirmed and thus this isotope is currently unknown or unconfirmed.
272Ds
In the first attempt to synthesize element 110, a 10 ms SF activity was assigned to 272Ds in the reaction 232Th(44Ca,4n). Given current understanding regarding stability, this isotope has been retracted from the Table of Isotopes.
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 267Ds ?? | 1994 | 209Bi(59Co,n) |
| 268Ds | unknown | |
| 269Ds | 1994 | 208Pb(62Ni,n) |
| 270Dsg,m | 2000 | 207Pb(64Ni,n) |
| 271Dsg,m | 1994 | 208Pb(64Ni,n) |
| 272Ds | unknown | |
| 273Ds | 1996 | 244Pu(34S,5n) |
| 274Ds | unknown | |
| 275Ds | unknown | |
| 276Ds | unknown | |
| 277Ds ?? | 1997 | 232Th(48Ca,3n) |
| 278Ds | unknown | |
| 279Ds | 2002 | 244Pu(48Ca,5n)[14] |
| 280Ds | unknown | |
| 281aDs | 1999 | 244Pu(48Ca,3n)[14] |
| 281bDs ? | 1999 | 244Pu(48Ca,3n)[14] |
Nuclear isomerism
281Ds
The production of 281Ds by the decay of 289Uuq or 293Uuh has produced two very conflicting decay modes. The most common and readily confirmed mode is SF with a half-life of 11 s. A much rarer and hitherto unconfirmed mode is alpha decay by emission of an 8.77 MeV alpha particle with an observed half-life of ~3.7 m. This decay is associated with a unique decay pathway from the parent nuclides and must be assigned to an isomeric level. The half-life suggests that it must be assigned to an isomeric state but further research is required to confirm these reports.
271Ds
Decay data from the direct synthesis of 271Ds clearly indicates the presence of two alpha groups. The first has alpha lines at 10.74 and 10.69 MeV with a half-life of 1.63 ms. The other has a single alpha line at 10.71 MeV with a half-life of 69 ms. The first has been assigned to the ground state and the latter to an isomeric level. It has been suggested that the closeness of the alpha decay energies indicates that the isomeric level may decay primarily by delayed gamma emission to the ground state, resulting in an identical measured alpha energy and a combined half-life for the two processes.
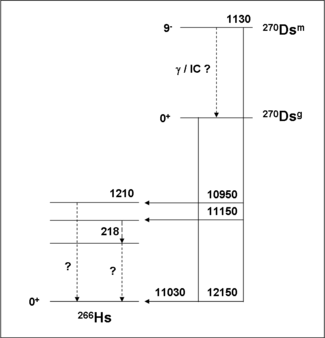
270Ds
The direct production of 270Ds has clearly identified two alpha groups belonging to two isomeric levels. The ground state decays into the ground state of 266Hs by emitting an 11.03 MeV alpha particle with a half-life of 0.10 ms. The isomeric level decays by alpha emission with alpha lines at 12.15,11.15 and 10.95 MeV with a half-life of 6 ms. The 12.15 MeV has been assigned as decay into the ground state of 266Hs indicating that this high spin K-isomer lies at 1.12 MeV above the ground state.
Spectroscopy
270Ds
Chemical yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing darmstadtium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n |
|---|---|---|---|---|---|
| 62Ni | 208Pb | 270Ds | 3.5 pb | ||
| 64Ni | 208Pb | 272Ds | 15 pb, 9.9 MeV |
Fission of compound nuclei with Z=110
Experiments have been performed in 2004 at the Flerov Laboratory of Nuclear Reactions in Dubna studying the fission characteristics of the compound nucleus 280Ds. The nuclear reaction used is 232Th+48Ca. The result revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82).[15]
Theoretical calculations
Decay characteristics
Theoretical calculation in a quantum tunneling model reproduces the experimental alpha decay half live data.[16][17] It also predicts that the isotope 294110 would have alpha decay half life of the order of 311 years.[18][19]
Evaporation residue cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.
DNS = Di-nuclear system; σ = cross section
| Target | Projectile | CN | Channel (product) | σmax | Model | Ref |
|---|---|---|---|---|---|---|
| 208Pb | 64Ni | 272Ds | 1n (271Ds) | 10 pb | DNS | [20] |
| 232Th | 48Ca | 280Ds | 4n (276Ds) | 0.2 pb | DNS | [21] |
| 230Th | 48Ca | 278Ds | 4n (274Ds) | 1 pb | DNS | [21] |
| 238U | 40Ar | 278Ds | 4n (274Ds) | 2 pb | DNS | [21] |
Chemical properties
Extrapolated chemical properties
Oxidation states
Darmstadtium is projected to be the eighth member of the 6d series of transition metals and the heaviest member of group 10 in the Periodic Table, below nickel, palladium and platinum. The highest confirmed oxidation state of +6 is shown by platinum whilst the +4 state is stable for both elements. Both elements also possess a stable +2 state. Darmstadtium is therefore predicted to show oxidation states +6, +4, and +2.
Chemistry
High oxidation states are expected to become more stable as the group is descended, so darmstadtium is expected to form a stable hexafluoride, DsF6, in addition to DsF5 and DsF4. Halogenation should result in the formation of the tetrahalides, DsCl4, DsBr4, and DsI4.
Like other Group 10 elements, darmstadtium can be expected to have notable hardness and catalytic properties.
See also
- Island of stability
References
- ↑ 1.0 1.1 Hofmann, S.; Ninov, V.; He�berger, F. P.; Armbruster, P.; Folger, H.; M�nzenberg, G.; Sch�tt, H. J.; Popeko, A. G. et al. (1995). "Production and decay of 269110". Zeitschrift für Physik a Hadrons and Nuclei 350: 277. doi:10.1007/BF01291181.
- ↑ 2.0 2.1 Hofmann, S (1998). Reports on Progress in Physics 61: 639. doi:10.1088/0034-4885/61/6/002.
- ↑ Karol, P. J.; Nakahara, H.; Petley, B. W.; Vogt, E. (2001). "On the discovery of the elements 110-112 (IUPAC Technical Report)". Pure and Applied Chemistry 73: 959. doi:10.1351/pac200173060959.
- ↑ ununnilium - Definitions from Dictionary.com
- ↑ Ginter, T. N.; Gregorich, K.; Loveland, W.; Lee, D.; Kirbach, U.; Sudowe, R.; Folden, C.; Patin, J. et al. (2003). "Confirmation of production of element 110 by the 208Pb(64Ni,n) reaction". Physical Review C 67: 064609. doi:10.1103/PhysRevC.67.064609.
- ↑ "Confirmation of production of element 110 by the 208Pb(64Ni,n) reaction", Ginter et al., LBNL repositories. Retrieved on 2008-03-02
- ↑ Folden, C. M.; Gregorich, KE; Düllmann, ChE; Mahmud, H; Pang, GK; Schwantes, JM; Sudowe, R; Zielinski, PM et al. (2004). "Development of an Odd-Z-Projectile Reaction for Heavy Element Synthesis: 208Pb(64Ni,n)271Ds and 208Pb(65Cu,n)272111". Physical Review Letters 93 (21): 212702. doi:10.1103/PhysRevLett.93.212702. PMID 15601003.
- ↑ "Development of an Odd-Z-Projectile Reaction for Heavy Element Synthesis: 208Pb(64Ni,n)271Ds and 208Pb(65Cu,n)272111", Folden et al., LBNL repositories. Retrieved on 2008-03-02
- ↑ Morita, K.; Morimoto, K.; Kaji, D.; Haba, H.; Ideguchi, E.; Kanungo, R.; Katori, K.; Koura, H. et al. (2004). "Production and decay of the isotope 271Ds (Z = 110)". The European Physical Journal A 21: 257. doi:10.1140/epja/i2003-10205-1.
- ↑ Hofmann et al; Heßberger, F.P.; Ackermann, D.; Antalic, S.; Cagarda, P.; Ćwiok, S.; Kindler, B.; Kojouharova, J. et al. (2001). "The new isotope 270110 and its decay products 266Hs and 262Sg". Eur. Phys. J. A 10: 5–10. doi:10.1007/s100500170137. http://www.dnp.fmph.uniba.sk/etext/68/text/Hofmann_et_al_EPJ_A10_(2001)_5.pdf.
- ↑ Ghiorso, A.; Lee, D.; Somerville, L.; Loveland, W.; Nitschke, J.; Ghiorso, W.; Seaborg, G.; Wilmarth, P. et al. (1995). "Evidence for the possible synthesis of element 110 produced by the 59Co+209Bi reaction". Physical Review C 51: R2293. doi:10.1103/PhysRevC.51.R2293.
- ↑ Lazarev, Yu. A.; Lobanov, Yu.; Oganessian, Yu.; Utyonkov, V.; Abdullin, F.; Polyakov, A.; Rigol, J.; Shirokovsky, I. et al. (1996). "α decay of 273110: Shell closure at N=162". Physical Review C 54: 620. doi:10.1103/PhysRevC.54.620.
- ↑ see ununoctium
- ↑ 14.0 14.1 14.2 see ununquadium
- ↑ see Flerov lab annual report 2004
- ↑ P. Roy Chowdhury, C. Samanta, and D. N. Basu (2006). "α decay half-lives of new superheavy elements". Phys. Rev. C 73: 014612. doi:10.1103/PhysRevC.73.014612.
- ↑ C. Samanta, P. Roy Chowdhury and D.N. Basu (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A 789: 142–154. doi:10.1016/j.nuclphysa.2007.04.001.
- ↑ P. Roy Chowdhury, C. Samanta, and D. N. Basu (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C 77: 044603. doi:10.1103/PhysRevC.77.044603.
- ↑ P. Roy Chowdhury, C. Samanta, and D. N. Basu (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". At. Data & Nucl. Data Tables 94: 781. doi:10.1016/j.adt.2008.01.003.
- ↑ Feng, Zhao-Qing; Jin, Gen-Ming; Li, Jun-Qing; Scheid, Werner (2007). "Formation of superheavy nuclei in cold fusion reactions". Physical Review C 76: 044606. doi:10.1103/PhysRevC.76.044606. http://arxiv.org/pdf/0707.2588.
- ↑ 21.0 21.1 21.2 Feng, Z; Jin, G; Li, J; Scheid, W (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A 816: 33. doi:10.1016/j.nuclphysa.2008.11.003. http://arxiv.org/pdf/0803.1117.
External links
- Darmstadtium.com
- WebElements.com: Darmstadtium
- IUPAC: Element 110 is named darmstadtium
- Apsidium: Darmstadtium 110
- [Eric Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, 2007]
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||