Keratin

Keratin refers to a family of fibrous structural proteins. Keratin is the key structural material making up the outer layer of human skin. It is also the key structural component of hair and nails. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and insoluble and form strong unmineralized tissues found in reptiles, birds, amphibians, and mammals. The only biological matter that rivals their toughness is chitin.
Contents |
Function
Keratin filaments are abundant in keratinocytes in the cornified layer of the epidermis; these are cells which have undergone keratinization.
- the α-keratins in the hair (including wool), horns, nails, claws and hooves of mammals
- the harder β-keratins found in nails and in the scales and claws of reptiles, their shells (chelonians, such as tortoise, turtle, terrapin), and in the feathers, beaks, claws of birds and quills of porcupines.[1] (These keratins are formed primarily in beta sheets. However, beta sheets are also found in α-keratins.)[2]
Arthropods such as crustaceans often have parts of their armor or exoskeleton made of keratin, sometimes in combination with chitin.
The baleen plates of filter-feeding whales are made of keratin.
It can be integrated in the chitinophosphatic matter that composes the shell and setae in many brachiopods.
Keratin is also found in the gastrointestinal tracts of many bioforms, including roundworms (which also have outer layers of keratin).
Although it is now difficult to be certain, the scales, claws, some protective armour and the beaks of dinosaurs would, almost certainly, have been composed of keratin.
Molecular biology and biochemistry
The usefulness of keratins depends on their supermolecular aggregation. These depend on the properties of the individual polypeptide strands, which depend in turn on their amino acid composition and sequence. The α-helix and β-sheet motifs, and disulfide bridges, are crucial to the conformations of globular, functional proteins like enzymes, many of which operate semi-independently, but they take on a completely dominant role in the architecture and aggregation of keratins.
The alpha keratin helix is not a true alpha helix, as it only has 3.5 residues/turn, where the normal alpha helix has 3.6 residues/turn. This is important for the different helices to form tight disulfide bonds. Also, roughly every seventh residue is a leucine, so they can line up and help the strands stick together through hydrophobic interactions.
Cornification
Cornification is the process of forming an epidermal barrier in stratified squamous epithelial tissue. At the cellular level, cornification is characterised by:
- production of keratin
- production of small proline-rich (SPRR) proteins and transglutaminase which eventually form a cornified cell envelope beneath the plasma membrane
- terminal differentiation
- loss of nuclei and organelles, in the final stages of cornification metabolism ceases and the cells are almost completely filled by keratin
During the process of epithelial differentiation, cells become cornified as keratin protein is incorporated into longer keratin intermediate filaments. Eventually the nucleus and cytoplasmic organelles disappear, metabolism ceases and cells undergo a programmed death as they become fully keratinized. In many other cell types, such as cells of the dermis, keratin filaments and other intermediate filaments function as part of the cytoskeleton to mechanically stabilize the cell against physical stress. It does this through connections to desmosomes, cell-cell junctional plaques, and hemidesmosomes, cell-basement membrane adhesive structures.
Cells in the epidermis contain a structural matrix of keratin, which makes this outermost layer of the skin almost waterproof, and along with collagen and elastin, gives skin its strength. Rubbing and pressure cause thickening of the outer, cornified layer of the epidermis and form protective calluses — useful for athletes and on the fingertips of musicians who play stringed instruments. Keratinized epidermal cells are constantly shed and replaced (see dandruff).
These hard, integumentary structures are formed by intercellular cementing of fibers formed from the dead, cornified cells generated by specialized beds deep within the skin. Hair grows continuously and feathers moult and regenerate. The constituent proteins may be phylogenetically homologous but differ somewhat in chemical structure and supermolecular organization. The evolutionary relationships are complex and only partially known. Multiple genes have been identified for the β-keratins in feathers, and this is probably characteristic of all keratins.
Glycine and alanine
Keratins contain a high proportion of the two smallest amino acids: glycine (whose "side group" is a single hydrogen atom) and alanine (with a small and noncharged methyl group). In the case of β-sheets, this allows sterically-unhindered hydrogen bonding between the amino and carboxyl groups of peptide bonds on adjacent protein chains, facilitating their close alignment and strong binding. Fibrous keratin molecules can twist around each other to form helical intermediate filaments.
Limited interior space is the reason why the triple helix of the (unrelated) structural protein collagen, found in skin, cartilage and bone, likewise has a high percentage of glycine. The connective tissue protein elastin also has a high percentage of both glycine and alanine. Silk fibroin, considered a β-keratin, can have these two as 75–80% of the total, with 10–15% serine, with the rest having bulky side groups. The chains are antiparallel, with an alternating C → N orientation.[3] A preponderance of amino acids with small, nonreactive side groups is characteristic for structural proteins, for which H-bonded close packing is more important than chemical specificity.
Disulfide bridges
In addition to intra- and intermolecular hydrogen bonds, keratins have large amounts of the sulfur-containing amino acid cysteine, required for the disulfide bridges that confer additional strength and rigidity by permanent, thermally-stable crosslinking—a role sulfur bridges also play in vulcanized rubber. Human hair is approximately 14% cysteine. The pungent smells of burning hair and rubber are due to the sulfur compounds formed. Extensive disulfide bonding contributes to the insolubility of keratins, except in dissociating or reducing agents.
The more flexible and elastic keratins of hair have fewer interchain disulfide bridges than the keratins in mammalian fingernails, hooves and claws (homologous structures), which are harder and more like their analogs in other vertebrate classes. Hair and other α-keratins consist of α-helically-coiled single protein strands (with regular intra-chain H-bonding), which are then further twisted into superhelical ropes that may be further coiled. The β-keratins of reptiles and birds have β-pleated sheets twisted together, then stabilized and hardened by disulfide bridges.
Filament formation
It used to be thought that keratins were being combined into 'hard' and 'soft,' or 'cytokeratins' and 'other keratins', but those designs are now understood to be correct. A new nuclear adition in 2006 to describe keratins takes this into account [4].
Keratin filaments are intermediate filaments. Like all intermediate filaments, keratin proteins form filamentous polymers in a series of assembly steps beginning with dimerization; dimers assemble into tetramers and octamers and eventually, the current hypothesis holds, into unit-length-filaments (ULF) capable of annealing end-to-end into long filaments.
Pairing
| A (neutral-basic) | B (acidic) | Occurrence |
| keratin 1, keratin 2 | keratin 9, keratin 10 | stratum corneum, keratinocytes |
| keratin 3 | keratin 12 | cornea |
| keratin 4 | keratin 13 | stratified epithelium |
| keratin 5 | keratin 14, keratin 15 | stratified epithelium |
| keratin 6 | keratin 16, keratin 17 | squamous epithelium |
| keratin 7 | keratin 19 | ductal epithelia |
| keratin 8 | keratin 18, keratin 20 | simple epithelium |
The entries KRT23, KRT24, KRT25, KRT26, KRT27, KRT28, KRT31, KRT32, KRT33, KRT33A, KRT34, KRT35, KRT36, KRT37, KRT38, KRT39, KRT40, KRT71, KRT72, KRT73, KRT74, KRT75, KRT76, KRT77, KRT78, KRT79, KRT8, KRT80, KRT81, KRT82, KRT83, KRT84, KRT85 and KRT86 have been used to describe keratins past 20.[5]
Silk
The silk fibroins produced by insects and spiders are often classified as keratins, though it is unclear whether they are phylogenetically related to vertebrate keratins.
Silk found in insect pupae, and in spider webs and egg casings, also has twisted β-pleated sheets incorporated into fibers wound into larger supermolecular aggregates. The structure of the spinnerets on spiders’ tails, and the contributions of their interior glands, provide remarkable control of fast extrusion. Spider silk is typically about 1 to 2 micrometres (µm) thick, compared with about 60 µm for human hair, and more for some mammals. The biologically and commercially useful properties of silk fibers depend on the organization of multiple adjacent protein chains into hard, crystalline regions of varying size, alternating with flexible, amorphous regions where the chains are randomly coiled.[6] A somewhat analogous situation occurs with synthetic polymers such as nylon, developed as a silk substitute. Silk from the hornet cocoon contains doublets about 10 µm across, with cores and coating, and may be arranged in up to 10 layers; also in plaques of variable shape. Adult hornets also use silk as a glue, as do spiders.
Clinical significance
Some infectious fungi, such as those that cause athlete's foot, ringworm, or the Batrachochytrium dendrobatidis (Chytrid fungus) which is killing amphibians all over the world, feed on keratin.
Diseases caused by mutations in the keratin genes include
- Epidermolysis bullosa simplex
- Ichthyosis bullosa of Siemens
- Epidermolytic hyperkeratosis
- Steatocystoma multiplex
- Keratosis pharyngis
- Rhabdoid cell formation in Large cell lung carcinoma with rhabdoid phenotype[7][8]
Furthermore, keratin expression is helpful in determining epithelial origin in anaplastic cancers. Tumors that express keratin include carcinomas, mesotheliomas, thymomas, sarcomas and trophoblastic neoplasms. Furthermore, the precise expression pattern of keratin subtypes allows prediction of the origin of the primary tumor when assessing metastases. For example, hepatocellular carcinomas typically expresse K8 and K18, and cholangiocarcinomas express K7, K8 and K18, while metastases of colorectal carcinomas express K20, but not K7.[9]
See also
- Keratosis pilaris
- Intermediate filament
- Desmosome
- Tinea versicolor
- Corneous
Additional images
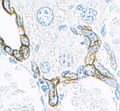 Keratin (high molecular weight) in bile duct cell and oval cells of horse liver. |
References
- ↑ Hickman, Cleveland Pendleton; Roberts, Larry S.; Larson, Allan L. (2003). Integrated principles of zoology. Dubuque, IA: McGraw-Hill. pp. 538. ISBN 0-07-243940-8.
- ↑ Kreplak L, Doucet J, Dumas P, Briki F (2004). "New aspects of the alpha-helix to beta-sheet transition in stretched hard alpha-keratin fibers". Biophys J 87 (1): 640–7. doi:10.1529/biophysj.103.036749. PMID 15240497.
- ↑ http://elmhurst.edu/~chm/vchembook/566secprotein.html
- ↑ Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006 Jul 17;174(2):169-74.
- ↑ Schweizer J, Bowden PE, Coulombe PA, et al. (July 2006). "New consensus nomenclature for mammalian keratins". J. Cell Biol. 174 (2): 169–74. doi:10.1083/jcb.200603161. PMID 16831889. PMC 2064177. http://www.jcb.org/cgi/pmidlookup?view=long&pmid=16831889.
- ↑ Spiders - Silk structure
- ↑ Shiratsuchi H, Saito T, Sakamoto A, Itakura E, Tamiya S, Oshiro Y, Oda Y, Toh S, Komiyama S, Tsuneyoshi M. Mutation analysis of human cytokeratin 8 gene in malignant rhabdoid tumor: a possible association with intracytoplasmic inclusion body formation. Mod Pathol 2002;15:146-53.
- ↑ Itakura E, Tamiya S, Morita K, Shiratsuchi H, Kinoshita Y, Oshiro Y, et al. Subcellular distribution of cytokeratin and vimentin in malignant rhabdoid tumor cells: three-dimensional imaging with confocal laser scanning microscopy and double immunofluorescence. Mod Pathol 2001; 14: 854–861.
- ↑ Omary MB, Ku NO, Strnad P, Hanada S (July 2009). "Toward unraveling the complexity of simple epithelial keratins in human disease". J. Clin. Invest. 119 (7): 1794–805. doi:10.1172/JCI37762. PMID 19587454. PMC 2701867. http://www.jci.org/articles/view/37762.
Bibliography
- Matsuda Y, Osaki T, Hashii T, Koshiba T, Kawabata S (November 2007). "A cysteine-rich protein from an arthropod stabilizes clotting mesh and immobilizes bacteria at injury sites". J. Biol. Chem. 282 (46): 33545–52. doi:10.1074/jbc.M705854200. PMID 17855345.
- Steinert PM, Kartasova T, Marekov LN (May 1998). "Biochemical evidence that small proline-rich proteins and trichohyalin function in epithelia by modulation of the biomechanical properties of their cornified cell envelopes". J. Biol. Chem. 273 (19): 11758–69. doi:10.1074/jbc.273.19.11758. PMID 9565599. http://www.jbc.org/cgi/pmidlookup?view=long&pmid=9565599.
- Ramot Y, Paus R, Tiede S, Zlotogorski A. Endocrine controls of keratin expression.
Bioessays. 2009 Apr;31(4):389-99. PMID: 19274655
External links
- Composition and β-sheet structure of silk
- Spider silk fiber structure
- Hair-Science.com's entry on the miroscopic elements of hair
- keratin antibody review
- silk fibroin
|
||||||||||||||||||||||||||||||