Cis–trans isomerism

In organic chemistry, cis-trans isomerism or geometric isomerism or configuration isomerism or E-Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule. In general, such isomers contain double bonds, which cannot rotate, but they can also arise from ring structures, wherein the rotation of bonds is greatly restricted. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes.
The terms cis and trans are from Latin, in which cis means "on the same side" and trans means "on the other side" or "across". The term "geometric isomerism" is considered an obsolete synonym of "cis-trans isomerism" by IUPAC[1]. It is sometimes used as a synonym for general stereoisomerism (e.g., optical isomerism being called geometric isomerism); the correct term for non-optical stereoisomerism is diastereomerism.
Contents |
Cis-trans isomerism in organic chemistry
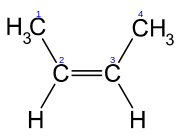
When the substituent groups are oriented in the same direction, the diastereomer is referred to as cis, whereas, when the substituents are oriented in opposing directions, the diastereomer is referred to as trans. An example of a small hydrocarbon displaying cis-trans isomerism is 2-butene.
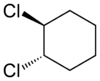
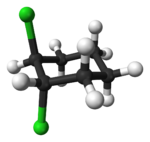
Alicyclic compounds can also display cis-trans isomerism. As an example of a geometric isomer due to a ring structure, consider 1,2-dichlorocyclohexane:
  |
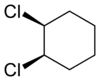 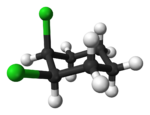 |
| trans-1,2-dichlorocyclohexane | cis-1,2-dichlorocyclohexane |
Physical properties - cis versus trans
Cis isomers and trans isomers often have different physical properties. Differences between isomers, in general, arise from the differences in the shape of the molecule or the overall dipole moment.
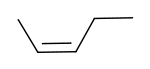 |
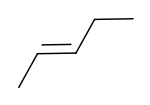 |
| cis-2-pentene | trans-2-pentene |
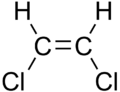 |
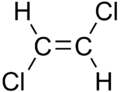 |
| cis-1,2-dichloroethene | trans-1,2-dichloroethene |
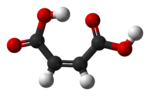 |
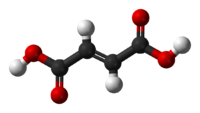 |
| cis-butenedioic acid (maleic acid) |
trans-butenedioic acid (fumaric acid) |
These differences can be very small, as in the case of the boiling point of straight-chain alkenes, such as 2-Pentene, which is 37°C in the cis isomer and 36°C in the trans isomer [1]. The differences between cis and trans isomers can be larger if polar bonds are present, as in the 1,2-dichloroethenes. The cis isomer in this case has a boiling point of 60.3°C, while the trans isomer has a boiling point of 47.5°C.[2] In the cis isomer the two polar C-Cl bond dipole moments combine to give an overall molecular dipole, so that there are intermolecular dipole-dipole forces which add to the London dispersion forces and raise the boiling point. In the trans isomer on the other hand, this does not occur because the two C-Cl bond moments cancel and the molecule is non-polar.
The two isomers of butenedioic acid have such large differences in properties and reactivities that they were actually given completely different names. The cis isomer is called maleic acid and the trans is named fumaric acid. Polarity is key in determining relative boiling point as it causes increased intermolecular forces, thereby raising the boiling point. In the same manner, symmetry is key in determining relative melting point as it allows for better packing in the solid state.
Thus, trans-alkenes which are less polar and more symmetrical have lower boiling points and higher melting points and cis-alkenes, which are generally more polar and less symmetrical have higher boiling points and lower melting points.
In the case of geometric isomers that are a consequence of double bonds, and, in particular, when both substituents are the same, some general trends usually hold. These trends can be attributed to the fact that the dipoles of the substituents in a cis isomer will add up to give an overall molecular dipole. In a trans isomer, the dipoles of the substituents will cancel out due to their being on opposite site of the molecule. Trans isomers also tend to have lower densities than their cis counterparts.
March[3] observes that, as trans alkenes, in general, have more symmetry than cis alkenes, the trans alkenes also tend to have higher melting points and lower solubility in inert solvents.
Vicinal coupling constants (3JHH), measured by NMR spectroscopy, are larger for trans- (range: 12–18 Hz, typical: 15 Hz) than for cis- (range: 0–12 Hz, typical: 8 Hz) isomers.[4]
Stability
Usually, trans isomers are more stable than the cis isomers. This is partly due to their shape; the straighter shape of the trans isomer leads to hydrogen intermolecular forces that make the isomer more stable . According to Jerry March, trans isomers also have a lower heat of combustion, indicating higher thermochemical stability. In the Benson Heat of formation group additivity, dataset cis isomers suffer a 1.10 kcal/mol stability penalty. Exceptions to this rule exist. For instance, for 1,2-difluoroethylene, 1,2-difluorodiazene (FN=NF), and several other halogen- and oxygen-substituted ethylenes. In this case, the cis isomer is more stable than the trans isomer.[5] This phenomenon is called the cis effect.[6]
E/Z notation
-1-bromo-1,2-dicholroethene.svg.png)
The cis/trans system for naming isomers is not effective when there are more than two different substituents on a double bond. The E/Z notation should then be used. Z (from the German zusammen) means together and corresponds to the term cis; E (from the German entgegen) means opposite and corresponds to the term trans.
Whether a molecular configuration is designated E or Z is determined by the Cahn-Ingold-Prelog priority rules (higher atomic numbers are given higher priority). For each of the two atoms in the double bond, it is necessary to determine which of the two substituents is of a higher priority. If both of the substituents of higher priority are on the same side, the arrangement is Z; if they are on opposite sides, the arrangement is E.
Inorganic coordination complexes
In inorganic coordination complexes with octahedral or square planar geometries, there are also cis isomers in which similar ligands are closer together and trans isomers in which they are further apart.

For example, there are two isomers of square planar Pt(NH3)2Cl2, as explained by Alfred Werner in 1893. The cis isomer, whose full name is cis-diamminedichloroplatinum(II), was shown in 1969 by Barnett Rosenberg to have antitumor activity and is now a chemotherapy drug known by the short name cisplatin. In contrast, the trans isomer (transplatin) has no useful anticancer activity. Each isomer can be synthesized using the trans effect to control which isomer is produced.
.png) |
.png) |
|
|
cis-[Co(NH3)4 Cl2]+ and trans-[Co(NH3)4 Cl2]+
|
||
For octahedral complexes of formula MX4Y2, two isomers also exist. (Here M is a metal atom, and X and Y are two different types of ligands.) In the cis isomer, the two Y ligands are adjacent to each other at 90o, as is true for the two chlorine atoms shown in green in cis-[Co(NH3)4Cl2]+, at left. In the trans isomer shown at right, the two Cl atoms are on opposite sides of the central Co atom.
A related type of isomerism in octahedral MX3Y3 complexes is facial-meridional (or fac-mer) isomerism, in which different numbers of ligands are cis or trans to each other.
See also
- E-Z notation
- Isomer
- Structural isomerism
- Chirality (chemistry)
- Trans fat
External links
- The IUPAC definition of "stereoisomerism"
- The IUPAC definition of "geometric isomerism"
- The IUPAC definition of "cis-trans isomers"
References
- ↑ Chemicalland values
- ↑ CRC Handbook of Chemistry and Physics, 60th Edition (1979-80), p.C-298
- ↑ Advanced organic Chemistry, Reactions, mechanisms and structure 3ed. page 111 Jerry March ISBN 0-471-85472-7
- ↑ "Spectroscopic Methods in Organic Chemistry," Dudly H. WIlliams and Ian FLeming, 4th ed. revised,McGraw-Hill Book COmpany (UK) Limited, 1989.Table 3.27
- ↑ The stereochemical consequences of electron delocalization in extended .pi. systems. An interpretation of the cis effect exhibited by 1,2-disubstituted ethylenes and related phenomena Richard C. Bingham J. Am. Chem. Soc.; 1976; 98(2); 535-540 Abstract
- ↑ Contribution to the Study of the Gauche Effect. The Complete Structure of the Anti Rotamer of 1,2-Difluoroethane Norman C. Craig et al. J. Am. Chem. Soc.; 1997; 119 p 4789 doi:10.1021/ja963819e