Chlorine
|
||||||||||||||||||||||||||||
| Appearance | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
pale yellow-green gas |
||||||||||||||||||||||||||||
| General properties | ||||||||||||||||||||||||||||
| Name, symbol, number | chlorine, Cl, 17 | |||||||||||||||||||||||||||
| Pronunciation | /ˈklɔəriːn/ KLOR-een | |||||||||||||||||||||||||||
| Element category | Halogen | |||||||||||||||||||||||||||
| Group, period, block | 17, 3, p | |||||||||||||||||||||||||||
| Standard atomic weight | 35.453g·mol−1 | |||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p5 | |||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 7 (Image) | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| Phase | gas | |||||||||||||||||||||||||||
| Density | (0 °C, 101.325 kPa) 3.2 g/L |
|||||||||||||||||||||||||||
| Liquid density at b.p. | 1.5625[1] g·cm−3 | |||||||||||||||||||||||||||
| Melting point | 171.6 K, -101.5 °C, -150.7 °F | |||||||||||||||||||||||||||
| Boiling point | 239.11 K, -34.04 °C, -29.27 °F | |||||||||||||||||||||||||||
| Critical point | 416.9 K, 7.991 MPa | |||||||||||||||||||||||||||
| Heat of fusion | (Cl2) 6.406 kJ·mol−1 | |||||||||||||||||||||||||||
| Heat of vaporization | (Cl2) 20.41 kJ·mol−1 | |||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) (Cl2) 33.949 J·mol−1·K−1 |
|||||||||||||||||||||||||||
| Vapor pressure | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Oxidation states | 7, 6, 5, 4, 3, 2, 1, -1 (strongly acidic oxide) |
|||||||||||||||||||||||||||
| Electronegativity | 3.16 (Pauling scale) | |||||||||||||||||||||||||||
| Ionization energies (more) |
1st: 1251.2 kJ·mol−1 | |||||||||||||||||||||||||||
| 2nd: 2298 kJ·mol−1 | ||||||||||||||||||||||||||||
| 3rd: 3822 kJ·mol−1 | ||||||||||||||||||||||||||||
| Covalent radius | 102±4 pm | |||||||||||||||||||||||||||
| Van der Waals radius | 175 pm | |||||||||||||||||||||||||||
| Miscellanea | ||||||||||||||||||||||||||||
| Crystal structure | orthorhombic | |||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[2] | |||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) > 10 Ω·m | |||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 8.9x10-3 W·m−1·K−1 | |||||||||||||||||||||||||||
| Speed of sound | (gas, 0 °C) 206 m/s | |||||||||||||||||||||||||||
| CAS registry number | 7782-50-5 | |||||||||||||||||||||||||||
| Most stable isotopes | ||||||||||||||||||||||||||||
| Main article: Isotopes of chlorine | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Chlorine (pronounced /ˈklɔ(ə)riːn/ KLOR-een, from the Greek word 'χλωρóς' (khlôros, meaning 'pale green'), is the chemical element with atomic number 17 and symbol Cl. It is a halogen, found in the periodic table in group 17 (formerly VII, VIIa, or VIIb). As the chloride ion, which is part of common salt and other compounds, it is abundant in nature and necessary to most forms of life, including humans. In its elemental form (Cl2 or "dichlorine") under standard conditions, chlorine is a powerful oxidant and is used in bleaching and disinfectants, as well as an essential reagent in the chemical industry. As a common disinfectant, chlorine compounds are used in swimming pools to keep them clean and sanitary. In the upper atmosphere, chlorine-containing molecules such as chlorofluorocarbons have been implicated in the destruction of the ozone layer.
Contents |
Characteristics
Physical characteristics
At standard temperature and pressure, two chlorine atoms form the diatomic molecule Cl2. This is a pale yellow-green gas that has its distinctive strong smell, the smell of bleach. The bonding between the two atoms is relatively weak (only 242.580 ±0.004 kJ/mol), which makes the Cl2 molecule highly reactive.
Chemical characteristics
Along with fluorine, bromine, iodine, and astatine, chlorine is a member of the halogen series that forms the group 17 of the periodic table. Chlorine forms compounds with almost all of the elements to give compounds that are usually called chlorides. Chlorine gas reacts with most organic compounds, and will even sluggishly support the combustion of hydrocarbons.[3]
Hydrolysis
At 10 °C and atmospheric pressure, one liter of water dissolves 3.10 L of gaseous chlorine,[4] Solutions of chlorine in water contain chlorine (Cl2), hydrochloric acid, and hypochlorous acid:
- Cl2 + H2O
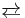 HCl + HClO
HCl + HClO
This conversion to the right is called disproportionation, because the ingredient chlorine both increases and decreases in formal oxidation state. The solubility of chlorine in water is increased if the water contains dissolved alkali hydroxide, and in this way, chlorine bleach is produced.
Compounds
Chlorine exists in all odd numbered oxidation states from −1 to +7, as well as the elemental state of zero (see Table). Progressing through the states, hydrochloric acid can be oxidized using manganese dioxide, or hydrogen chloride gas oxidized catalytically by air to form elemental chlorine gas.
| Oxidation state |
Name | Formula | Illustrative compounds |
|---|---|---|---|
| −1 | chlorides | Cl− | ionic chlorides, organic chlorides, hydrochloric acid |
| 0 | chlorine | Cl2 | elemental chlorine |
| +1 | hypochlorites | ClO− | sodium hypochlorite, calcium hypochlorite |
| +3 | chlorites | ClO−2 | sodium chlorite |
| +4 | chlorine dioxide | ClO2 | |
| +5 | chlorates | ClO−3 | sodium chlorate, potassium chlorate, chloric acid |
| +7 | perchlorates | ClO−4 | perchloric acid, perchlorate salts such as magnesium perchlorate, dichlorine heptoxide |
Interhalogen compounds
Chlorine oxidizes bromide and iodide salts to bromine and iodine, respectively. But it cannot oxidize fluoride to fluorine. It makes are variety of "interhalogen compounds" such as the chlorine fluorides, chlorine monofluoride (ClF), chlorine trifluoride (ClF3), chlorine pentafluoride (ClF5). Chlorides of bromine and iodine are also known.
Organo chlorine compounds
Chlorine is used extensively in organic in substitution and addition reactions. Chlorine often imparts many desired properties to an organic compound, in part due to its electronegativity. Organochlorine compounds are also serious pollutants, either as side products of industrial processes or as persistent pesticidess.
Many important industrial products are produced via organochlorine intermediates. Examples include polycarbonates, polyurethanes, silicones, polytetrafluoroethylene, carboxymethyl cellulose, and propylene oxide. Like the other halogens, chlorine participates in free-radical substitution reactions with hydrogen-containing organic compounds. When applied to organic substrates, reaction is often—but not invariably—non-regioselective, and, hence, may result in a mixture of isomeric products. It is often difficult to control the degree of substitution as well, so multiple substitutions are common. If the different reaction products are easily separated, e.g., by distillation, substitutive free-radical chlorination (in some cases accompanied by concurrent thermal dehydrochlorination) may be a useful synthetic route. Industrial examples of this are the production of methyl chloride, methylene chloride, chloroform, and carbon tetrachloride from methane, allyl chloride from propylene, and trichloroethylene, and tetrachloroethylene from 1,2-dichloroethane.
Like the other halides, chlorine undergoes electrophilic additions reactions, the most notable one being the chlorination of alkenes and aromatic compounds with a Lewis acid catalyst. Organic chlorine compounds tend to be less reactive in nucleophilic substitution reactions than the corresponding bromine or iodine derivatives, but they tend to be cheaper. They may be activated for reaction by substituting with a tosylate group, or by the use of a catalytic amount of sodium iodide.
Chlorides
Chlorine combines with almost all elements to give chlorides. Compounds with oxygen, nitrogen, xenon, and krypton are known, but do not form by direct reaction of the elements.[5] Chloride is one of the most common anions in nature. Hydrogen chloride and its aqueous solution, hydrochloric acid, are produced on megaton scale annually both as valued intermediates but sometimes as undesirable pollutants.
Chlorine oxides
Chlorine forms a variety of oxides: chlorine dioxide (ClO2), dichlorine monoxide (Cl2O), dichlorine heptoxide (Cl2O7). The anionic derivatives of these same oxides are also well known including chlorate (ClO−3), chlorite (ClO−2), hypochlorite (ClO−), and perchlorate (ClO−4), and chloramine (NH2Cl).[6] The acid derivatives of these anions are hypochlorous acid (HOCl), chloric acid (HClO3) and perchloric acid (HClO4).
In hot concentrated alkali solution hypochlorite disproportionates:
- 2 ClO− → Cl− + ClO−2
- ClO− + ClO−2 → Cl− + ClO−3
Sodium chlorate and potassium chlorate can be crystallized from solutions formed by the above reactions. If their crystals are heated, they undergo a further, final disproportionation:
- 4 ClO−3 → Cl− + 3 ClO−4
This same progression from chloride to perchlorate can be accomplished by electrolysis. The anode reaction progression is:[7]
-
Reaction Electrode
potentialCl− + 2 OH− → ClO− + H2O + 2 e− +0.89 volts ClO− + 2 OH− → ClO−2 + H2O + 2 e− +0.67 volts ClO−2 + 2 OH− → ClO−3 + H2O + 2 e− +0.33 volts ClO−3 + 2 OH− → ClO−4 + H2O + 2 e− +0.35 volts
Each step is accompanied at the cathode by
- 2 H2O + 2 e− → 2 OH− + H2 (−0.83 volts)
Occurrence
See also Category: Halide minerals In nature, chlorine is found primarily as the chloride ion, a component of the salt that is deposited in the earth or dissolved in the oceans — about 1.9% of the mass of seawater is chloride ions. Even higher concentrations of chloride are found in the Dead Sea and in underground brine deposits. Most chloride salts are soluble in water, thus, chloride-containing minerals are usually only found in abundance in dry climates or deep underground. Common chloride minerals include halite (sodium chloride), sylvite (potassium chloride), and carnallite (potassium magnesium chloride hexahydrate). Over 2000 naturally occurring organic chlorine compounds are known.[8]
In the interstellar medium, chlorine is produced in supernovae via the r-process.[9]
Isotopes
Chlorine has a wide range of isotopes, the two principal stable isotopes being 35Cl (75.77%) and 37Cl (24.23%); they give chlorine atoms an apparent atomic weight of 35.4527 g/mol.
Trace amounts of radioactive 36Cl exist in the environment, in a ratio of about 7x10−13 to 1 with stable isotopes. 36Cl is produced in the atmosphere by spallation of 36Ar by interactions with cosmic ray protons. In the subsurface environment, 36Cl is generated primarily as a result of neutron capture by 35Cl or muon capture by 40Ca. 36Cl decays to 36S and to 36Ar, with a combined half-life of 308,000 years. The half-life of this hydrophilic nonreactive isotope makes it suitable for geologic dating in the range of 60,000 to 1 million years. Additionally, large amounts of 36Cl were produced by irradiation of seawater during atmospheric detonations of nuclear weapons between 1952 and 1958. The residence time of 36Cl in the atmosphere is about 1 week. Thus, as an event marker of 1950s water in soil and ground water, 36Cl is also useful for dating waters less than 50 years before the present. 36Cl has seen use in other areas of the geological sciences, including dating ice and sediments.
History

The most common compound of chlorine, sodium chloride, has been known since ancient times; archaeologists have found evidence that rock salt was used as early as 3000 BC and brine as early as 6000 BC.[10]
Elemental chlorine was first prepared and studied in 1774 by Swedish chemist Carl Wilhelm Scheele, and, therefore, he is credited for its discovery.[11] He called it "dephlogisticated muriatic acid air" since it is a gas (then called "airs") and it came from hydrochloric acid (then known as "muriatic acid").[11] However, he failed to establish chlorine as an element, mistakenly thinking that it was the oxide obtained from the hydrochloric acid (see phlogiston theory).[11] He named the new element within this oxide as muriaticum.[11] Regardless of what he thought, Scheele did isolate chlorine by reacting MnO2 (as the mineral pyrolusite) with HCl:
- 4 HCl + MnO2 → MnCl2 + 2 H2O + Cl2
Scheele observed several of the properties of chlorine: the bleaching effect on litmus, the deadly effect on insects, the yellow green color, and the smell similar to aqua regia.
At the time, common chemical theory was: Any acid is a compound that contains oxygen (still sounding in the German and Dutch names of oxygen: sauerstoff or zuurstof, both translating into English as acid stuff), so a number of chemists, including Claude Berthollet, suggested that Scheele's dephlogisticated muriatic acid air must be a combination of oxygen and the yet undiscovered element, muriaticum.[12]
In 1809, Joseph Louis Gay-Lussac and Louis-Jacques Thénard tried to decompose dephlogisticated muriatic acid air by reacting it with charcoal to release the free element muriaticum (and carbon dioxide).[11] They did not succeed and published a report in which they considered the possibility that dephlogisticated muriatic acid air is an element, but were not convinced.[13]
In 1810, Sir Humphry Davy tried the same experiment again, and concluded that it is an element, and not a compound.[11] He named this new element as chlorine, from the Greek word χλωρος (chlōros), meaning green-yellow.[14] The name halogen, meaning salt producer, was originally defined for chlorine (in 1811 by Johann Salomo Christoph Schweigger), and it was later applied to the rest of the elements in this family.[15] In 1823, Michael Faraday liquefied chlorine for the first time.[16][17]
Chlorine was first used to bleach textiles in 1785.[18] In 1826, silver chloride was used to produce photographic images for the first time.[19] Chloroform was first used as an anesthetic in 1847.[19] A chlorine solution in lime-water (hypochlorite) was first used as a germicide to prevent the spread of puerperal fever in the maternity wards of Vienna General Hospital in Austria in 1847,[20] and in 1850 by John Snow to disinfect the water supply in London after an outbreak of cholera. The US Department of Treasury called for all water to be disinfected with chlorine by 1918.[19] Polyvinylchloride (PVC) was invented in 1912, initially without a purpose.[19] Chlorine gas was first introduced as a weapon on April 22, 1915 at Ypres by the German Army,[21][22] and the results of this weapon were disastrous because gas masks had not yet been invented.
Production
In industry, elemental chlorine is usually produced by the electrolysis of sodium chloride dissolved in water. Along with chlorine, this chloralkali process yields hydrogen gas and sodium hydroxide, according to the following chemical equation:
- 2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH
The electrolysis of chloride solutions all proceed according to the following equations:
- Cathode: 2 H+ (aq) + 2 e− → H2 (g)
- Anode: 2 Cl− (aq) → Cl2 (g) + 2 e−
Overall process: 2 NaCl (or KCl) + 2 H2O → Cl2 + H2 + 2 NaOH (or KOH) In diaphragm cell electrolysis, an asbestos (or polymer-fiber) diaphragm separates a cathode and an anode, preventing the chlorine forming at the anode from re-mixing with the sodium hydroxide and the hydrogen formed at the cathode.[23] The salt solution (brine) is continuously fed to the anode compartment and flows through the diaphragm to the cathode compartment, where the caustic alkali is produced and the brine is partially depleted. Diaphragm methods produce dilute and slightly impure alkali but they are not burdened with the problem of preventing mercury discharge into the environment and they more energy efficient. Membrane cell electrolysis employ permeable membrane as an ion exchanger. Saturated sodium (or potassium) chloride solution is passed through the anode compartment, leaving at a lower concentration.[24] This method is more efficient than the diaphragm cell and produces very pure sodium (or potassium) hydroxide at about 32% concentration, but requires very pure brine.

Laboratory methods
Small amounts of chlorine gas can be made in the laboratory by combining hydrochloric acid and manganese dioxide. Alternatively a strong acid such as sulfuric acid or hydrochloric acid reacts with sodium hypochlorite or sodium chlorate solution to release chlorine gas. In the home, accidents occur when hypochlorite bleach solutions are combined with certain acidic drain-cleaners.
Applications
Production of industrial and consumer products
Chlorine's principal applications are in the production of a wide range of industrial and consumer products.[25][26] For example, it is used in making plastics, solvents for dry cleaning and metal degreasing, textiles, agrochemicals and pharmaceuticals, insecticides, dyestuffs, household cleaning products, etc.
Purification and disinfection
Chlorine is an important chemical for water purification (such as water treatment plants), in disinfectants, and in bleach. Chlorine in water is more than three times more effective as a disinfectant against Escherichia coli than an equivalent concentration of bromine, and is more than six times more effective than an equivalent concentration of iodine.[27]
Chlorine is usually used (in the form of hypochlorous acid) to kill bacteria and other microbes in drinking water supplies and public swimming pools. In most private swimming pools, chlorine itself is not used, but rather sodium hypochlorite, formed from chlorine and sodium hydroxide, or solid tablets of chlorinated isocyanurates. Even small water supplies are now routinely chlorinated.[3] (See also chlorination)
It is often impractical to store and use poisonous chlorine gas for water treatment, so alternative methods of adding chlorine are used. These include hypochlorite solutions, which gradually release chlorine into the water, and compounds like sodium dichloro-s-triazinetrione (dihydrate or anhydrous), sometimes referred to as "dichlor", and trichloro-s-triazinetrione, sometimes referred to as "trichlor". These compounds are stable while solid and may be used in powdered, granular, or tablet form. When added in small amounts to pool water or industrial water systems, the chlorine atoms hydrolyze from the rest of the molecule forming hypochlorous acid (HOCl), which acts as a general biocide, killing germs, micro-organisms, algae, and so on.
Use as a weapon
- World War I
Chlorine gas, also known as bertholite, was first used as a weapon in World War I by Germany on April 22, 1915 in the Second Battle of Ypres. As described by the soldiers it had a distinctive smell of a mixture between pepper and pineapple. It also tasted metallic and stung the back of the throat and chest. Chlorine can react with water in the mucosa of the lungs to form hydrochloric acid, an irritant that can be lethal. The damage done by chlorine gas can be prevented by a gas mask, or other filtration method, which makes the overall chance of death by chlorine gas much lower than those of other chemical weapons. It was pioneered by a German scientist later to be a Nobel laureate, Fritz Haber of the Kaiser Wilhelm Institute in Berlin, in collaboration with the German chemical conglomerate IG Farben, who developed methods for discharging chlorine gas against an entrenched enemy. It is alleged that Haber's role in the use of chlorine as a deadly weapon drove his wife, Clara Immerwahr, to suicide. After its first use, chlorine was utilized by both sides as a chemical weapon, but it was soon replaced by the more deadly gases phosgene and mustard gas.[28]
- Iraq War
Chlorine gas has also been used by insurgents against the local population and coalition forces in the Iraq War in the form of chlorine bombs. On March 17, 2007, for example, three chlorine filled trucks were detonated in the Anbar province killing two and sickening over 350.[29] Other chlorine bomb attacks resulted in higher death tolls, with more than 30 deaths on two separate occasions.[30] Most of the deaths were caused by the force of the explosions rather than the effects of chlorine, since the toxic gas is readily dispersed and diluted in the atmosphere by the blast. The Iraqi authorities have tightened up security for chlorine, which is essential for providing safe drinking water for the population.
Chlorine cracking

The element is widely used for purifying water owing to its powerful oxidizing properties, especially potable water supplies and water used in swimming pools. Several catastrophic collapses of swimming pool ceilings have occurred owing to stress corrosion cracking of stainless steel rods used to suspend them.[31] Some polymers are also sensitive to attack, including acetal resin and polybutene. Both materials were used in hot and cold water domestic supplies, and stress corrosion cracking caused widespread failures in the USA in the 1980s and 1990s. One example shows an acetal joint in a water supply system, which, when it fractured, caused substantial physical damage to computers in the labs below the supply. The cracks started at injection molding defects in the joint and grew slowly until finally triggered. The fracture surface shows iron and calcium salts that were deposited in the leaking joint from the water supply before failure.
Other uses
Chlorine is used in the manufacture of numerous organic chlorine compounds, the most significant of which in terms of production volume are 1,2-dichloroethane and vinyl chloride, intermediates in the production of PVC. Other particularly important organochlorines are methyl chloride, methylene chloride, chloroform, vinylidene chloride, trichloroethylene, perchloroethylene, allyl chloride, epichlorohydrin, chlorobenzene, dichlorobenzenes, and trichlorobenzenes.
Chlorine is also used in the production of chlorates and in bromine extraction.
Mapping of industrial releases in the United States
One tool that maps the most recent release information of chlorine [1] to particular locations in the United States[32] and also provides additional information about such releases is TOXMAP. TOXMAP is a Geographic Information System (GIS) from the Division of Specialized Information Services of the United States National Library of Medicine (NLM) that uses maps of the United States to help users visually explore data from the United States Environmental Protection Agency's (EPA) Toxics Release Inventory and Superfund Basic Research Programs. TOXMAP is a resource funded by the US Federal Government. TOXMAP's chemical and environmental health information is taken from NLM's Toxicology Data Network (TOXNET)[33] and PubMed, and from other authoritative sources.
Health effects
| NFPA 704 |
|---|
Chlorine is a toxic gas that irritates the respiratory system. Because it is heavier than air, it tends to accumulate at the bottom of poorly ventilated spaces. Chlorine gas is a strong oxidizer, which may react with flammable materials.[34]
Chlorine is detectable in concentrations of as low as 0.2 ppm. Coughing and vomiting may occur at 30 ppm and lung damage at 60 ppm. About 1000 ppm can be fatal after a few deep breaths of the gas.[4] Breathing lower concentrations can aggravate the respiratory system, and exposure to the gas can irritate the eyes.[35] The toxicity of chlorine comes from its oxidizing power. When chlorine is inhaled at concentrations above 30 ppm, it begins to react with water and cells, which change it into hydrochloric acid (HCl) and hypochlorous acid (HClO).
When used at specified levels for water disinfection, the reaction of chlorine with water is not a major concern for human health. However, other materials present in the water may generate disinfection by-products that can damage human health.[36][37]
See also
- Polymer degradation
References
- ↑ Chlorine, Gas Encyclopaedia, Air Liquide
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics 81st edition, CRC press.
- ↑ 3.0 3.1 Hammond, C. R. (2000). The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 0849304814.
- ↑ 4.0 4.1 "WebElements.com – Chlorine". Mark Winter [The University of Sheffield and WebElements Ltd, UK]. http://www.webelements.com/webelements/elements/text/Cl/index.html. Retrieved 2007-03-17.
- ↑ Windholz, Martha et al., ed (1976). Merck Index of Chemicals and Drugs, 9th ed.. Rahway, N.J.: Merck & Co.. ISBN 0911910263.
- ↑ "Chlorine compounds of the month". Euro Chlor. http://www.eurochlor.org/index.asp?page=678. Retrieved 2007-08-29.
- ↑ Cotton, F. Albert and Wilkinson, Geoffrey (1966). Advanced Inorganic Chemistry, 2nd ed.. John Wiley & sons. p. 568.
- ↑ "Risk assessment and the cycling of natural organochlorines". Euro Chlor. http://www.eurochlor.org/upload/documents/document236.pdf. Retrieved 2007-08-12.
- ↑ A.G.W. Cameron (June 1957). "Stellar Evolution, Nuclear Astrophysics, and Nucleogenesis". CRL-41. "http://www.fas.org/sgp/eprint/CRL-41.pdf"
- ↑ "The earliest salt production in the world: an early Neolithic exploitation in Poiana Slatinei-Lunca, Romania". http://antiquity.ac.uk/ProjGall/weller/. Retrieved 2008-07-10.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 "17 Chlorine". Elements.vanderkrogt.net. http://elements.vanderkrogt.net/elem/cl.html. Retrieved 2008-09-12.
- ↑ Ihde, Aaron John (1984). The development of modern chemistry. Courier Dover Publications. p. 158. ISBN 0486642356. http://books.google.com/?id=34KwmkU4LG0C&pg=PA158.
- ↑ Gay-Lussac, Joseph Louis; Thénard, Louis-Jacques (1809). "On the nature and the properties of muriatic acid and of oxygenated muriatic acid". Mémoires de Physique et de Chimie de la Société d'Arcueil 2: 339–358. http://web.lemoyne.edu/~giunta/thenard.html.
- ↑ Sir Humphry Davy (1811). "On a Combination of Oxymuriatic Gas and Oxygene Gas". Philosophical Transactions of the Royal Society 101: 155–162. doi:10.1098/rstl.1811.0008. http://www.chemteam.info/Chem-History/Davy-Chlorine-1811.html.
- ↑ Snelders, H. A. M. (1971). "J. S. C. Schweigger: His Romanticism and His Crystal Electrical Theory of Matter". Isis 62 (3): 328. doi:10.1086/350763. http://www.jstor.org/pss/229946.
- ↑ "This Month in Physics History September 4, 1821 and August 29, 1831: Faraday and Electromagnetism". http://www.aps.org/publications/apsnews/200108/history.cfm. Retrieved 2010-05-08.
- ↑ "Michael Faraday". http://www-history.mcs.st-andrews.ac.uk/Biographies/Faraday.html. Retrieved 2010-05-08.
- ↑ "History of Chlorine". http://members.aol.com/manbio999/chlorine.htm. Retrieved 2008-07-10.
- ↑ 19.0 19.1 19.2 19.3 Jacqueline Brazin. "Chlorine & its Consequences". Archived from the original on September 18, 2006. http://web.archive.org/web/20060918145109/http://ocw.mit.edu/NR/rdonlyres/Earth--Atmospheric--and-Planetary-Sciences/12-091January--IAP--2006/0EF9264B-3205-44A3-8306-8E8364917DF0/0/brazin.pdf. Retrieved 2008-07-10.
- ↑ "Chlorine Story". americanchemistry. http://www.americanchemistry.com/s_chlorine/sec_content.asp?CID=1166&DID=4476&CTYPEID=109. Retrieved 2008-07-10.
- ↑ "Chlorine - History". http://www.drcordas.com/education/weaponsmassd/Chlorine.pdf. Retrieved 2008-07-10.
- ↑ "Weaponry: Use of Chlorine Gas Cylinders in World War I". historynet.com. http://www.historynet.com/weaponry-use-of-chlorine-gas-cylinders-in-world-war-i.htm. Retrieved 2008-07-10.
- ↑ "Diaphragm cell". Euro Chlor. http://www.eurochlor.org/animations/diaphragm-cell.asp. Retrieved 2007-08-15.
- ↑ "Membrane cell". Euro Chlor. http://www.eurochlor.org/animations/membrane-cell.asp. Retrieved 2007-08-15.
- ↑ "Uses". Euro Chlor. http://www.eurochlor.org/uses. Retrieved 2007-08-20.
- ↑ "Chlorine Tree". Chlorine Tree. http://www.chlorinetree.org. Retrieved 2007-08-20.
- ↑ Koski TA, Stuart LS, Ortenzio LF (1966). "Comparison of chlorine, bromine, iodine as disinfectants for swimming pool water". Applied Microbiology 14 (2): 276–279. PMID 4959984. PMC 546668. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=4959984.
- ↑ "Weapons of War: Poison Gas". First World War.com. http://www.firstworldwar.com/weaponry/gas.htm. Retrieved 2007-08-12.
- ↑ Mahdi, Basim (2007-03-17). "Iraq gas attack makes hundreds ill". CNN. http://www.cnn.com/2007/WORLD/meast/03/17/iraq.main/index.html. Retrieved 2007-03-17.
- ↑ "'Chlorine bomb' hits Iraq village". BBC News. 2007-05-17. http://news.bbc.co.uk/2/hi/middle_east/6660585.stm. Retrieved 2007-05-17.
- ↑ Bertolini, Luca; Elsener, Bernhard; Pedeferri, Pietro; Polder, Rob B. (2004). Corrosion of steel in concrete: prevention, diagnosis, repair. Wiley-VCH. p. 148. ISBN 3527308008. http://books.google.com/?id=cEmq232h1zcC&pg=PA148.
- ↑ "TRI Releases Map". Toxmap.nlm.nih.gov. http://toxmap.nlm.nih.gov/toxmap/. Retrieved 2010-03-23.
- ↑ TOXNET - Databases on toxicology, hazardous chemicals, environmental health, and toxic releases
- ↑ "Chlorine MSDS". October 23, 1997 (Revised November 1999. http://www.westlake.com/datasheets/MSDS_Chlorine.pdf.
- ↑ Winder, Chris (2001). "The Toxicology of Chlorine". Environmental Research 85 (2): 105–114. doi:10.1006/enrs.2000.4110. PMID 11161660.
- ↑ "What's in your Water?: Disinfectants Create Toxic By-products". ACES News. College of Agricultural, Consumer and Environmental Sciences - University of Illinois at Urbana-Champaign. 2009-03-31. http://www.aces.uiuc.edu/news/stories/news4724.html. Retrieved 2009-03-31.
- ↑ PMID 17980649 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
External links
- Agency for Toxic Substances and Disease Registry: Chlorine
- Electrolytic production
- Production and liquefaction of chlorine
- Chlorine Production Using Mercury, Environmental Considerations and Alternatives
- National Pollutant Inventory - Chlorine
- National Institute for Occupational Safety and Health - Chlorine Page
- WebElements.com — Chlorine
- Chlorine Institute - Trade association and lobby group representing the interests of the chlorine industry
- Chlorine Online - Chlorine Online is an information resource produced by Eurochlor - the business association of the European chlor-alkali industry
|
|||||
|
|||||
|
|||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
|||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
