Boletus edulis
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
Boletus edulis, commonly known as penny bun, porcino or cep, is a basidiomycete fungus, and the type species of the genus Boletus. Widely distributed in the Northern Hemisphere across Europe, Asia, and North America, it does not occur naturally in the Southern Hemisphere, although it has been introduced to southern Africa and New Zealand. Several closely related European mushrooms formerly thought to be varieties or forms of B. edulis have been shown using molecular phylogenetic analysis to be distinct species. The western North American species commonly known as the California king bolete (Boletus edulis var. grandedulis) is a large, darker-coloured variant that was first formally identified in 2008.
The fungus grows in deciduous and coniferous forests and tree plantations, forming symbiotic ectomycorrhizal associations with living trees by enveloping sheaths of fungal tissue around their underground roots. The fungus produces spore-bearing fruit bodies above ground in summer and autumn. The fruit body consists of a large and imposing brown cap which on occasion can reach at least 35 cm (14 in) in diameter and 3 kg (6.6 lb) in weight. Like other boletes, it has tubes extending downward from the underside of the cap, rather than gills; spores are released at maturity through the tube openings, or pores. The pore surface of the B. edulis fruit body is whitish when young, but ages to a greenish-yellow. The stout stipe, or stem, is white or yellowish in colour, up to 25 cm (10 in) tall and 10 cm (3.9 in) thick, and partially covered with a raised network pattern, or reticulations.
Prized as an ingredient in various foods, B. edulis is an edible mushroom held in high regard in many cuisines, and is commonly prepared and eaten in soups, pasta, or risotto. The mushroom is low in fat and digestible carbohydrates, and high in protein, vitamins, minerals and dietary fibre. Although it is sold commercially, it has not been successfully grown in cultivation. Available fresh in autumn in Central, Southern and Northern Europe, it is most often dried, packaged and distributed worldwide. Keeping its flavour after drying, it is then reconstituted and used in cooking. Boletus edulis is one of the few fungi that are sold pickled. The fungus also produces a variety of organic compounds with a diverse spectrum of biological activity, including the steroid derivative ergosterol, a sugar binding protein, antiviral compounds, antioxidants, and phytochelatins, which give the organism resistance to toxic heavy metals.
Contents |
Taxonomy

Boletus edulis was first described in 1782 by the French botanist Pierre Bulliard and still bears its original name.[1] The starting date of fungal taxonomy had been set as January 1, 1821, to coincide with the date of the works of the 'father of mycology', Swedish naturalist Elias Magnus Fries, which meant that the name required sanction by Fries (indicated in the name by a colon) to be considered valid, as Bulliard's work preceded this date. It was thus written Boletus edulis Bull.:Fr. However, a 1987 revision of the International Code of Botanical Nomenclature set the starting date at May 1, 1753, the date of publication of Linnaeus' seminal work, the Species Plantarum.[2] Hence the name no longer requires the ratification of Fries' authority. Early alternate names include Boletus solidus by English naturalist James Sowerby in 1809,[3] and Gray's Leccinum edule.[4] Gray's transfer of the species to Leccinum was later determined to be inconsistent with the rules of botanical nomenclature, and it is apparent that he was unfamiliar with the earlier works of Fries when he published his arrangement of bolete species.[5]
Boletus edulis is the type species of the genus Boletus. In Rolf Singer's classification of the Agaricales mushrooms, it is also the type species of section Boletus, a grouping of about 30 related boletes united by a number of characteristics: a mild-tasting white flesh that does not change colour when exposed to air; a smooth to distinctly raised netted pattern over at least the uppermost portion of the stem; a yellow-brown or olive-brown spore print; white tubes that later become yellowish then greenish, and that initially appear to be stuffed with cotton; and cystidia that are not strongly coloured.[6][7] Molecular analysis published in 2006 established that the bolete mushrooms are all derived from a common ancestor, and established the Boletales as an order separate from the Agaricales.[8]
The first named species of the genus Boletus, the generic name is derived from the Latin term bōlētus "mushroom", which was borrowed in turn from the Ancient Greek βωλίτης, "terrestrial fungus".[9] Ultimately, this last word derives from bōlos/βῶλος "lump", "clod", and, metaphorically, "mushroom".[10] However, the βωλίτης of Galen, like the boletus of Latin writers like Martial, Seneca and Petronius,[11] is often identified as the much prized Amanita caesarea.[12] The specific epithet edulis in Latin means "eatable" or "edible".[13]
Common names
Common names for Boletus edulis vary by region. The standard Italian name porcino means "piglet" in Italian, and echoes the term suilli, literally 'hog mushrooms', used by the Ancient Romans,[14] and still surviving in Southern Italian words for this mushroom.[15] The derivation has been ascribed to the resemblance of young fruit bodies to piglets, or to the fondness pigs have for eating them.[16] It is also known as king bolete.[17] The English penny bun refers to its rounded brownish shape. The German name Steinpilz "stone mushroom" is derived from the firm flesh.[18] In Austria it is called Herrenpilz, the "gentlemen's mushroom",[16] while in Mexico, the Spanish name is panza, meaning "belly".[19] Another Spanish name, rodellon, means "small round boulder", while the Dutch name eekhoorntjes brood means "squirrel's bread". [20] In Albanian it is called pankushe or barkushe, the first name probably deriving from Latin and the second one its analog in Albanian meaning "the belly one" from bark=belly. Its Polish name prawdziwek stems from 'prawda' or truth, suggesting that it is the "true mushroom", reflecting its status as the king of field mushrooms in that country.
The vernacular name cep is derived from the Catalan cep or its French name cèpe, although the latter is a generic term applying to several species. In France, it is more fully cèpe de Bordeaux, derived from the Gascon cep "trunk" for its fat stalk,[21] ultimately from the Latin cippus "stake".[22] Ceppatello, ceppatello buono, ceppatello bianco, giallo leonato, ghezzo, and moreccio are names from Italian dialects,[23][24] and ciurenys or surenys is another term in Catalan.[25] The French-born King Charles XIV John popularised B. edulis in Sweden after 1818,[26] and is honoured in the local vernacular name Karljohanssvamp. The monarch cultivated the fungus about his residence, Rosersberg Palace.[27] It is known as hed tab tao เห็ดตับเต่า in Thai.[28]
Description

The cap of this mushroom is 7–30 cm (3–12 in) broad at maturity. Slightly sticky to touch, it is convex in shape when young and flattens with age. The colour is generally reddish-brown fading to white in areas near the margin, and continues to darken as it matures. The stipe, or stem, is 8–25 cm (3.5–10 in) in height, and up to 7 cm (2.8 in) thick—rather large in comparison to the cap;[29] it is club-shaped, or bulges out in the middle. It is finely reticulate on the upper portion, but smooth or irregularly ridged on the lower part. The under surface of the cap is made of thin tubes, the site of spore production; they are 1 to 2 cm (0.4 to 0.8 in) deep, and whitish in colour when young, but mature to a greenish-yellow.[30] The angular pores, which do not stain when bruised, are small — roughly 2 to 3 pores per millimetre.[31] In youth the pores are white and appear as if stuffed with cotton (which is actually mycelia); as they age, they change colour to yellow and later to brown. The spore print is olive brown. The flesh of the fruit body is white, thick and firm when young, but becomes somewhat spongy with age. When bruised or cut, it either does not change colour, or turns a very light brown.[32] Fully mature specimens can weigh about 1 kg (2.2 lb); a huge specimen collected on the Isle of Skye, Scotland, in 1995 bore a cap of 42 cm (16.5 in), with a stipe 18 cm (7.1 in) in height and 14 cm (5.5 in) wide, and weighed 3.2 kg (7.1 lb).[29]
.jpg) |
 |
|
|
Stem shape can range from club-shaped to centrally bulbous
|
||
Boletus edulis is considered one of the safest wild mushrooms to pick for the table, as there are no poisonous species that closely resemble it.[16] The most similar mushroom may be the devil's bolete (Boletus satanas), which has a similar shape, but has a red stem and stains blue on bruising.[16] However, it is often confused with the very bitter and unpalatable Tylopilus felleus, but can be distinguished by the reticulation on the stalk; in porcini, it is a whitish net-like pattern on a brownish stalk, whereas it is a dark pattern on white in the latter. Porcini have whitish pores while the other has pink. If in doubt, tasting a tiny bit of flesh will yield a bitter taste.[16] It can also resemble the "bolete-like" Gyroporus castaneus, which is generally smaller, and has a browner stem.
The spores are elliptical to spindle-shaped, with dimensions of 12–17 by 5–7 µm. The basidia, the spore-bearing cells, are produced in a layer lining the tubes, and arrange themselves so their ends are facing the center of the tube; this layer of cells is known technically as a hymenium. The basidia are thin-walled, mostly attached to 4 spores, and measure 25–30 by 8–10 µm. Another cell type present in the hymenium are the cystidia, larger sterile cells that protrude beyond the basidia into the lumen of the hymenium, and act as air traps, regulating humidity.[33] B. edulis has pleurocystidia (cystidia located on the face of a pore) that are thin-walled, roughly spindle-shaped to ventricose, and measure 30–45 by 7–10 µm; the hymenium does not have cheilocystidia — cells found on the edges of the pores.[30] The hyphae of B. edulis do not have clamp connections.[31]
Related species
 |
 |
|
|
B. edulis var. grandedulis
|
B. regineus
|
Several similar brownish-coloured species are sometimes considered subspecies or forms of this mushroom. In Europe, in addition to B. edulis (or cèpe de Bordeaux), the most popular are:
- Tête de nègre ("negro’s head"; Boletus aereus), much rarer than B. edulis, is more highly regarded by gourmets, and more expensive. Usually smaller than B. edulis, it is also distinctively darker in colour.[16] It is especially suited to drying.[17]
- Cèpe des pins ("pine tree cep"; Boletus pinophilus or Boletus pinicola) grows among pine trees. Rarer than B. edulis, it is less appreciated by gourmets than the two other kinds of porcini, but remains a mushroom rated above most others.[17]
- Cèpe d'été ("summer cep"; Boletus reticulatus), also less common and found earlier.[16]
Molecular phylogenetic analyses have proven that these three are all distinctive and separate species;[34] other taxa formerly believed to be unique species or subspecies, such as B. betulicola, B. persoonii, B. quercicola and B. venturii, are now known to be part of a B. edulis species complex with a wide morphological, ecological and geographic range.[35] Similar molecular technology has been developed to rapidly and accurately identify B. edulis and other commercially important fungi.[36][37]

Western North America has a number of species closely related to B. edulis. The white king bolete (Boletus barrowsii), found in parts of Colorado, New Mexico, Arizona, and California (and possibly elsewhere), is named after its discoverer Chuck Barrows.[38] It is lighter in colour than B. edulis, having a cream-coloured cap with pink tones;[39] often mycorrhizal with Ponderosa pine, it tends to grow in areas where there is less rainfall. Some find its flavour as good as if not better than B. edulis.[40] The California king bolete (Boletus edulis var. grandedulis) can reach massive proportions, and is distinguished from B. edulis by a mature pore surface that is brown to slightly reddish. The cap colour appears to be affected by the amount of light received during its development, and may range from white in young specimens grown under thick canopy, to dark-brown, red-brown or yellow brown in those specimens receiving more light.[41] The queen bolete (Boletus regineus), formerly considered a variety of B. aereus, is also a choice edible. It is generally smaller than B. edulis, and unlike that species, is typically found in mixed forests.[42] The spring king bolete (Boletus rex-veris), formerly considered a variety of B. edulis or B. pinophilus, is found throughout western North America. In contrast to B. edulis, B. rex-veris tends to fruit in clusters, and, as its common name suggests, appears in the spring.[43]
Habitat and distribution

The fruit bodies of Boletus edulis can grow singly or in small clusters of two or three specimens. The mushroom's habitat consists of areas dominated by pine (Pinus spp.), spruce (Picea spp.), hemlock (Tsuga spp.) and fir (Abies spp.) trees, although other hosts include chestnut, chinquapin, beech, Keteleeria spp., Lithocarpus spp., and oak. In California, porcini have been collected in a variety of forests, such as coastal forests, dry interior oak forests and savannas and interior high-elevation montane mixed forests,[44] to an altitude of 3,500 m (11,500 ft).[45] In northwestern Spain, they are common in scrublands dominated by the rock rose species Cistus ladanifer and Halimium lasianthum.[46]
Boletus edulis has a cosmopolitan distribution, concentrated in cool-temperate to subtropical regions.[44] It is common in Europe—from northern Scandinavia, south to the extremities of Greece and Italy—and North America, where its southern range extends as far south as Mexico.[32] It is well known from the Borgotaro area of Parma, Italy, and has PGI status there. The European distribution extends north to Scandinavia and south to southern Italy and Morocco.[44] In China, the mushroom can be found from the northeastern Heilongjiang Province to the Yunnan-Guizhou Plateau and Tibet.[32] It has been recorded growing under Pinus and Tsuga in Sagarmatha National Park in Nepal,[47] as well as in the Indian forests of Arunachal Pradesh.[48]
Non-native introductions
Boletus edulis grows in some areas where it is not believed to be indigenous. It is often found underneath oak and silver birch in Hagley Park in central Christchurch, New Zealand, where it is likely to have been introduced,[49] probably on the roots of container-grown beech, birch, and oak in the mid 1800s—around the time exotic trees began to be planted in the Christchurch area.[32] It has been growing plentifully in association with pine forests in the southern KwaZulu-Natal Midlands in South Africa for more than 50 years and is believed to have been introduced with the import of pine trees.[50][51] It also grows in pine plantations in neighboring Zimbabwe.[52]
Ecology


Fruit body production
Although Italian folklore holds that porcini sprout up at the time of the new moon,[16] a number of research studies have tried to investigate more scientifically the factors that influence the production of fruit bodies. Although fruit bodies may appear any time from summer to autumn, their growth is known to be triggered by rainfall during warm periods of weather followed by frequent autumn rain with a drop in soil temperature.[44] Above average rainfall may result in the rapid appearance of large numbers of boletes, in what is known in some circles as a "bolete year".[53] A 2004 field study indicated that fruit body production is enhanced by an open and sunny wood habitat,[54] corroborating an earlier observation made in a Zimbabwean study;[52] removal of the litter layer on the forest floor appeared to have a negative effect on fruit body production, but previous studies reported contradictory results.[55][56] A Lithuanian study conducted in 2001 concluded that the maximal daily growth rate of the cap (about 21 mm or 0.8 in) occurred when the relative air humidity was the greatest, and the fruit bodies ceased growing when the air humidity dropped below 40%. Factors most likely to inhibit the appearance of fruit bodies included prolonged drought, inadequate air and soil humidity, sudden decreases of night air temperatures, and the appearance of the first frost.[57] Plots facing north tend to produce more mushrooms compared to equivalent plots facing south.[58]
Mycorrhizal associations
Boletus edulis is mycorrhizal—it is in a mutualistic relationship with the roots of plants ("hosts"), in which the fungus exchanges minerals extracted from the environment for fixed carbon from the host. Other benefits for the plant are evident: in the case of the Chinese Chestnut, the formation of mycorrhizae with B. edulis increases the ability of plant seedlings to resist water stress, and increases leaf succulence, leaf area, and water-holding ability.[59] The fungus forms a sheath of tissue around terminal, nutrient-absorbing rootlets of the host, forming so-called "ectomycorrhizae"; the fungal hyphae emanate throughout the soil, effectively increasing the surface area for nutrient absorption, and the fungus penetrates between cells of the cortex to facilitate nutrient exchange. Compatible hosts may belong to multiple families of vascular plants that are widely distributed throughout the Northern Hemisphere; according to one 1995 estimate, there are at least 30 host plant species distributed over more than 15 genera.[32] Examples of mycorrhizal associates include Chinese red pine,[60] Mexican weeping pine,[61] Scots pine, Norway spruce,[62] Coast Douglas-fir,[63] mountain pine,[64] and Virginia pine.[65] The fungus has also been shown to associate with Gum rockrose, a pioneer early stage shrub that is adapted for growth in degraded areas, such as burned forests.[66] These and other Rockrose species are ecologically important as fungal reservoirs, maintaining an inoculum of mycorrhizal fungi for trees that appear later in the forest regrowth cycle.[67]
The mushroom has been noted to commonly co-occur with Amanita muscaria or A. rubescens, although it is unclear whether this is due to a biological association between the species, or because of similarities in growing season, habitat, and ecological requirements.[44] An association has also been reported between B. edulis and Amanita excelsa on Pinus radiata ectomycorrhizae in New Zealand, suggesting that other fungi may influence the life cycle of porcini.[68] A 2007 field study revealed little correlation between the abundance of fruit bodies and presence of its mycelia below ground, even when soil samples were taken from directly beneath the mushroom; the study concluded "Factors triggering formation of mycorrhizae and fructification of porcini appear to be too complex to be simply explained on the basis of the amount of fungal mycelia in the soil."[69]
Heavy metal contamination
Boletus edulis is known to be able to tolerate and even thrive on soil that is contaminated with toxic heavy metals, such as soil that might be found near metal smelters. The mushroom's resistance to heavy metal toxicity is conferred by a biochemical called a phytochelatin—an oligopeptide whose production is induced after exposure to metal.[70] Phytochelatins are chelating agents, capable of forming multiple bonds with the metal; in this state, the metal cannot normally react with other elements or ions and is stored in a detoxified form in the mushroom tissue.
Pests and predators
The fruit bodies of B. edulis can be infected by the parasitic mould-like fungus Hypomyces chrysospermus, known as the bolete eater, which manifests itself as a white, yellow, or reddish-brown cottony layer over the surface of the mushroom.[71] Some reported cases of stomach ache following consumption of dried porcini have been attributed to the presence of this mould on the fruit bodies.[72] The mushroom is also used as a food source by several species of mushroom flies,[44] as well as other insects and their larvae.[73] An unidentified species of virus was reported to have infected specimens found in the Netherlands and in Italy; fruit bodies affected by the virus had relatively thick stems and small or no caps, leading to the name "little-cap disease".[74][75]
Boletus edulis is a food source for animals such as the banana slug (Ariolimax columbianus),[76] the Long-haired Grass Mouse,[77] the Red Squirrel,[78] and, as noted in one isolated report, the Fox Sparrow.[79]
Culinary uses
Boletus edulis, like its name implies, is an edible mushroom. Italian chef and restaurateur Antonio Carluccio has described it as representing "the wild mushroom par excellence", and hails it as the most rewarding of all fungi in the kitchen for its taste and versatility.[16] Considered a choice edible, particularly in France, Germany and Italy,[17] it was widely written about by the Roman writers Pliny the Elder and Martial, although ranked below the esteemed Amanita caesarea.
sunt tibi boleti:fungos ego sumo suillos (Ep. iii. 60)
("You eat the choice boletus, I have mushrooms that swine grub up.")[80]
wrote the disgruntled Martial when served suilli instead of boleti.[81] The term suilli was also thought to encompass the related Leccinum scabrum.[82]
The flavour has been described as nutty and slightly meaty, with a smooth, creamy texture, and a distinctive aroma reminiscent of sourdough. Young, small porcini are most appreciated by gourmets, as the large ones often harbor maggots (insect larvae), and become slimy, soft and less tasty with age. Fruit bodies are collected by holding the stipe near the base and twisting gently. Cutting the stipe with a knife may risk the part left behind rotting and the mycelium being destroyed. Peeling and washing are not recommended.[16] The fruit bodies are highly perishable, due largely to the high water content (around 90%), the high level of enzyme activity, and the presence of a flora of microorganisms.[83] Caution should be exercised when collecting specimens from potentially polluted or contaminated sites, as several studies have shown that the fruit bodies can bioaccumulate toxic heavy metals like mercury,[84] cadmium,[85] caesium and polonium.[86][87] Bioaccumulated metals or radioactive fission decay products are like chemical signatures: chemical and radiochemical analysis can be used to identify the origin of imported specimens,[88] and for long-term radioecological monitoring of polluted areas.[89]
Porcini are sold fresh in markets in summer and autumn in Central and Southern Europe, and dried or canned at other times of the year, and distributed worldwide to countries where they are not otherwise found.[90] They are eaten and enjoyed raw, sautéed with butter, ground into pasta, in soups, and in many other dishes. In France, they are used in recipes such as cèpes à la Bordelaise, cèpe frits and cèpe aux tomates.[91] Porcini risotto is a traditional Italian autumn dish.[92] Porcini are a feature of many cuisines, including Provençal,[93] and Viennese.[94] They are used in soups and consumed blanched in salads in Thailand.[28] Porcini can also be frozen — either raw or first cooked in butter. The colour, aroma, and taste of frozen porcini deteriorate noticeably if frozen longer than four months. Blanching or soaking and blanching as a processing step before freezing can extend the freezer life up to 12 months.[83] They are also one of the few mushroom species pickled and sold commercially.[95]
Dried

Boletus edulis is well suited to drying — its flavour intensifies, it is easily reconstituted, and its resulting texture is pleasant.[96] Dried porcini have more protein than most other commonly consumed vegetables apart from soybeans. However, some of this content is indigestible, though digestibility is improved with cooking.[97]
Like other boletes, porcini can be dried by being strung separately on twine and hung close to the ceiling of a kitchen. Alternatively, the mushrooms can be dried by cleaning with a brush (washing is not recommended), and then placing them in a wicker basket or bamboo steamer on top of a boiler or hot water tank.[98] Another method is drying in an oven at 25 to 30 °C (77 to 86 °F) for two to three hours, then increasing the temperature to 50 °C (122 °F) until crisp or brittle.[99] Once dry, they are kept in an airtight jar.[98] Importantly for commercial production, porcini retain their flavour after industrial preparation in a pressure cooker or after canning or bottling, and are thus useful for manufacturers of soups or stews. The addition of a few pieces of dried porcino can significantly add to flavour, and they are a major ingredient of the pasta sauce known as carrettiere (carter's sauce).[100] The drying process is known to induce the formation of various volatile substances that contribute to the mushroom's aroma. Chemical analysis has shown that the odour of the dried mushroom is a complex mixture of 53 volatile compounds.[101]
Commercial harvest

A 1998 estimate suggests the total annual worldwide consumption of Boletus edulis and closely related species (B. aereus, B. pinophilus, and B. reticulatus) to be between 20,000 and 100,000 tons.[44] Approximately 2,700 tonnes (3,000 tons) were sold in France, Italy and Germany in 1988, according to official figures. The true amount consumed far exceeds this, as it does not account for informal sales or consumption by collectors.[45] They are widely exported and sold in dried form, reaching countries where they do not occur naturally, such as Australia and New Zealand. The autonomous community of Castile and León in Spain produces 7,700 tonnes (8,500 tons) annually.[66] In autumn, the price of porcini in the Northern Hemisphere typically ranges between $20 and $80 dollars per kilogram, although in New York in 1997, the scarcity of fruit bodies elevated the wholesale price to over $200 per kilogram.[45]
In the vicinity of Borgotaro in the Province of Parma of northern Italy, the four species Boletus edulis, B. aereus, B. aestivalis and B. pinophilus have been recognised for their superior taste and officially termed Fungo di Borgotaro. Here, these mushrooms have been collected for centuries, and exported commercially. However, due to recent trends in the globalization of mushroom trade, most of the porcini commercially available in Italy or exported by Italy no longer originate from Italy. Porcini and other mushrooms are imported into Italy from various locations, especially China and eastern European countries; these are then often re-exported under the "Italian porcini" label.[102][103]
In Italy, the disconnect with local production has had an adverse effect on quality; for example, in the 1990s, some of the dried porcino mushrooms exported to Italy from China contained species of genus Tylopilus, which are rather similar in appearance, and when dried, are difficult for both mushroom labourers and mycologists alike to distinguish from Boletus. Tylopilus species typically have a very bitter taste, a bitterness that is imparted to the flavour of the porcini with which they are mixed.[104]
After the fall of the Iron Curtain and the economic and political barriers that followed, central and eastern European countries with local mushroom harvesting traditions, such as Albania, Bulgaria, Macedonia, Romania, Serbia and Slovenia, developed into exporters of porcini, concentrating primarily on the Italian market.[103] Exported porcini and other wild fungi are also destined for France, Germany and other western European markets, where demand for them exists, but collection on a commercial scale does not.[103] Picking B. edulis has become an annual seasonal income earner and pastime in countries like Bulgaria, especially for many Roma communities and the unemployed.[105] Unfortunately, a lack of control has led to heavy exploitation of the mushroom resource.[106]
As with other strictly mycorrhizal fungi, B. edulis has eluded attempts to cultivate it.[97][107] The results of some studies suggest that unknown components of the soil microflora might be required for B. edulis to successfully establish a mycorrhizal relationship with the host plant.[108][109][110]
Nutritional composition
| Nutritional value per 100 g (3.5 oz) | |
|---|---|
| Energy | 342.4 kJ (81.8 kcal) |
| Fat | 1.70 g |
| Protein | 7.39 g |
| Thiamine (Vit. B1) | 0.105 mg (8%) |
| Riboflavin (Vit. B2) | 0.092 mg (6%) |
| Niacin (Vit. B3) | 6.07 mg (40%) |
| Pantothenic acid (B5) | 2.64 mg (53%) |
| Vitamin B6 | 0.051 mg (4%) |
| Folate (Vit. B9) | 290 μg (73%) |
| Vitamin C | 4.21 mg (7%) |
| Calcium | 1.195 mg (0%) |
| Iron | 0.739 mg (6%) |
| Phosphorus | 22.26 mg (3%) |
| Potassium | 203.3 mg (4%) |
| Zinc | 4.172 mg (42%) |
| Percentages are relative to US recommendations for adults. Source: USDA Nutrient database |
|
Boletus edulis constitutes a food source which, although not rich in easily absorbed carbohydrates or fat, contains vitamins, minerals and dietary fibre. Fresh mushrooms contain over 80% moisture,[112] although reported values tend to differ somewhat as moisture content can be affected by environmental temperature and relative humidity during growth and storage, as well as the relative amount of water that may be produced as a result of normal metabolic processes during storage.[113]
Carbohydrates make up the bulk of the fruit bodies, comprising 9.23% of the fresh weight (see table), and 65.4% of the dry weight.[112] The carbohydrate component contains the monosaccharides glucose, mannitol and α,α-trehalose, the polysaccharide glycogen, and the water-insoluble structural polysaccharide chitin, which accounts for up to 80–90% of dry matter in mushroom cell walls. Chitin, hemicellulose, and pectin-like carbohydrates—all indigestible by humans—contribute to the nutritionally desirable high proportion of insoluble fibre in B. edulis.[114]
The total lipid, or crude fat, content makes up 2.6% of the dry matter of the mushroom. The proportion of fatty acids (expressed as a % of total fatty acids) are: palmitic acid, 9.8%; stearic acid, 2.7%; oleic acid, 36.1%; linoleic acid, 42.2%, and linolenic acid, 0.2%.[115][nb 1]
A comparative study of the amino acid composition of eleven Portuguese wild edible mushroom species showed Boletus edulis to have the highest total amino acid content,[nb 2] about 2.3 g per 100 g of dried mushroom. This total includes a full complement of 20 essential and nonessential amino acids.[116] Analysis of the free amino acids (that is, those not bound up in protein) revealed glutamine and alanine to be the principal amino acids (each about 25% of total compounds); a separate analysis concluded that lysine is another predominant compound.[117]
Reported values of the composition and concentrations of trace metals and minerals in Boletus edulis tend to differ considerably, as the mushroom bioaccumulates different elements to varying degrees, and the element concentration in the fruit bodies is often a reflection of the element concentration of the soils from which they were picked.[114] In general, B. edulis contains appreciable amounts of selenium, a trace mineral essential for good health;[118] however, the bioavailability of mushroom-derived selenium is low.[119] Whole fruit bodies also contain about 200 μg of vitamin D2 per 100 g dry weight.[120] The relatively high ergosterol content (see next section) of the fruit bodies can make the mushroom nutritionally pragmatic for vegetarians and vegans, who would otherwise have a limited intake of vitamin D from foods of animal origin.[114]
Bioactive compounds
Boletus edulis fruit bodies contain about 500 mg of ergosterol per 100 g of dried mushroom.[120] Ergosterol is a sterol compound common in fungi. Additionally, the fruit bodies have about 30 mg of ergosterol peroxide per 100 g of dried mushroom. Ergosterol peroxide is a steroid derivative with a wide spectrum of biological activity, including antimicrobial and anti-inflammatory activity, and cytotoxicity to various tumor cell lines grown in laboratory culture.[121]
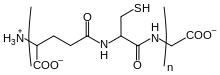
The mushroom also contains a sugar-binding protein, or lectin, that has affinity for the sugars xylose and melibiose. The lectin is mitogenic—that is, it can stimulate cells to begin the process of cell division, resulting in mitosis. Further, the lectin has antiviral properties: it inhibits the human immunodeficiency virus enzyme reverse transcriptase.[122] Other studies suggest that B. edulis also has antiviral activity against Vaccinia virus[123] and tobacco mosaic virus grown in culture.[124] Antiviral compounds from mushrooms are a subject of interest in biomedical research for their potential to advance the knowledge of viral replication, and as new drugs in the treatment of viral disease.[125]
The fruit bodies have a high antioxidative capacity, due probably to a combination of various organic acids (such as oxalic, citric, malic, succinic and fumaric acids), phenolic compounds and alkaloids; the highest antioxidative capacity is in the mushroom caps.[126] Furthermore, fruit bodies were determined to have 528 mg of the antioxidant compound ergothioneine per kilogram of fresh mushroom; this value was the highest among many food items tested in one study.[127] Porcini were thought to have anticancer properties according to Hungarian research conducted in the 1950s,[128] but later investigations in the United States did not support this.[90]
See also
- List of Boletus species
Notes
- ↑ A comparable Indian analysis produced somewhat different values: total lipids, 3.3% of dry matter; palmitic acid, 21.6%; stearic acid, 9.1%; oleic acid, 31.1%; linoleic acid, 33.8%, and linolenic acid, 1.7%. Source: Kavishree S, Hemavathy J, Lokesh BR, Shashirekha MN, Rajarathnam S. (2008). "Fat and fatty acids in Indian edible mushrooms". Food Chemistry 106: 597–602.
- ↑ Other species tested were Suillus bellinii, Suillus luteus, Suillus granulatus, Tricholomopsis rutilans, Hygrophorus agathosmus, Amanita rubescens, Russula cyanoxantha, Tricholoma equestre, Fistulina hepatica, and Cantharellus cibarius.
References
- ↑ Bulliard JBF. (1782) (in French). Herbier de la France. Vol 2. Paris, France: P.F. Didot. pp. 49–96, plate 60. http://www.archive.org/stream/herbierdelafranc4996bull#page/22/mode/2up. Retrieved 2009-11-24.
- ↑ Esser K, Lemke PA. (1994). The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research. Heidelberg, Germany: Springer. p. 81. ISBN 3540664939.
- ↑ Sowerby J. (1809). Coloured Figures of English Fungi. Volume 4. London: J. Davis. p. 199. This entire work is available in Commons here, but the reference is to plate 419 with textual description on page 697. However Sowerby described the same modern species as edulis on plate 111 (description on page 57).
- ↑ Gray SF. (1821). A Natural Arrangement of British Plants. London: Baldwin, Cradock, and Joy, Paternoster-Row. p. 647. http://books.google.com/?id=g-EYAAAAYAAJ&dq=A%20natural%20arrangement%20of%20British%20plants%20Gray%201821&pg=PA647#v=onepage&q=. Retrieved 2009-11-24.
- ↑ Šutara J. (1985). "Leccinum and the question of superfluous names (Fungi: Boletaceae)". Taxon 34 (4): 678–86. doi:10.2307/1222214. http://jstor.org/stable/1222214.
- ↑ Singer R. (1986). The Agaricales in Modern Taxonomy. (4th rev. ed.). Koenigstein, Germany: Koeltz Scientific Books. p. 779. ISBN 3-87429-254-1.
- ↑ Smith AH, Thiers HD. (1971). The Boletes of Michigan. Ann Arbor, Michigan: University of Michigan Press. p. 221. http://quod.lib.umich.edu/cgi/t/text/pageviewer-idx?c=fung1tc;cc=fung1tc;idno=agk0838.0001.001;frm=frameset;view=image;seq=229;page=root;size=s. Retrieved 2010-12-02.
- ↑ Binder M, Hibbett DS. (2006). "Molecular systematics and biological diversification of Boletales" (PDF). Mycologia 98 (6): 971–81. doi:10.3852/mycologia.98.6.971. PMID 17486973. http://www.mycologia.org/cgi/reprint/98/6/971.pdf. Retrieved 2009-11-03.
- ↑ Simpson DP. (1979). Cassell's Latin Dictionary (5 ed.). London: Cassell Ltd. p. 883. ISBN 0-304-52257-0.
- ↑ Liddell HG, Scott R. (1980). A Greek-English Lexicon (Abridged ed.). United Kingdom: Oxford University Press. ISBN 0-19-910207-4.
- ↑ Peter Howell, A Commentary on Book One of the Epigrams of Martial, The Athlone Press, 1980 p.152-3. Howell doubts the identification, and mentions the view advanced by Augusta A.Imholtz Jr., 'Fungi and piace- names, thè origin of boletus,' in AJP Vol.98, 1977 pp.71f., that the Latin word may derive from the Spanish town Boletum, modern-day Boltaña, south of the Pyrenees, which is still famous for its mushrooms.
- ↑ Ramsbottom J. (1953). Mushrooms & Toadstools. London, England: Collins. p. 6. ISBN 1870630092.
- ↑ Jamieson A, Ainsworth R, Morell T. (1828). Latin Dictionary: Morell's Abridgment. London: Moon, Boys & Graves. pp. 121, 596. http://books.google.com/?id=U3oTAAAAYAAJ&printsec=frontcover&dq=latin+dictionary. Retrieved 2009-11-02.
- ↑ Pliny, Natural History, Bk.16, 11, 31: 'Such is the multiplicity of products in addition to the acorn that are borne by hard-oaks; but they also produce edible fungi (boletos) and hog-mushrooms (suillosque).' (Pliny, Natural History, 10 vols., tr.H. Rackham, Harvard University Press/Heinemann, (1945) 1968, vol.4, pp. 408–409.
- ↑ Neapolitan sillo, and Calabrian sillu/siddu. See Glauco Sanga, Gherardo Ortalli, Nature knowledge: ethnoscience, cognition, and utility, Berghahn Books, 2003 p. 78.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 16.8 16.9 Carluccio, pp. 36–38.
- ↑ 17.0 17.1 17.2 17.3 Zeitlmayr L. (1976). Wild Mushrooms: An Illustrated Handbook. Hertfordshire, UK: Garden City Press. p. 96. ISBN 0-584-10324-7.
- ↑ Grimm J., Grimm W. (1838–1961). Deutsches Wörterbuch. Leipzig: Hirzel. (online)
- ↑ Jarvis MC, Miller AM, Sheahan J, Ploetz K, Ploetz J, Watson RR, Ruiz MP, Villapan CAP, Alvarado JG, Ramirez AL, Orr B. (2004). "Edible wild mushrooms of the Cofre de Perote region, Veracruz, Mexico: An ethnomycological study of common names and uses". Economic Botany 58 (Suppl. S): S111–S115. doi:10.1663/0013-0001(2004)58[S111:EWMOTC]2.0.CO;2.
- ↑ Schalkwijk-Barendsen HME. (1991). Mushrooms of Western Canada. Edmonton: Lone Pine Publishing. p. 195. ISBN 0-919433-47-2.
- ↑ Grigson J. (1975). The Mushroom Feast. London: Penguin. p. 8. ISBN 0-14-046-273-2.
- ↑ J. Simpson, E. Weiner (eds), ed (1989). "cepe". Oxford English Dictionary (2nd edition ed.). Oxford: Clarendon Press. ISBN 0-19-861186-2.
- ↑ Naccari NL. (1827). Flora veneta. 4–6. L. Bonvecchiato. p. 10.
- ↑ Zeitlmayr L. (1977). I funghi. Edizioni Studio Tesi. p. 180.
- ↑ Andrews, Colman (1999) [1988]. Catalan cuisine: vivid flavors from Spain's Mediterranean coast (2nd ed.). Boston, MA: Harvard Common Press. p. 88. ISBN 1-55832-329-5.
- ↑ Spoerke DG, Rumack BH. (1994). Handbook of Mushroom Poisoning: Diagnosis and Treatment. Boca Raton, Florida: CRC Press. p. 11. ISBN 0849301947.
- ↑ Stensaas M, Sonstegard J. (2004). Canoe Country Flora: Plants and Trees of the North Woods and Boundary Waters. Minneapolis, Minnesota: University of Minnesota Press. p. 189. ISBN 1570251215.
- ↑ 28.0 28.1 Solomon C. (1996). Charmaine Solomon's Encyclopedia of Asian Food. Melbourne: William Heinemann Australia. p. 238. ISBN 0-85561-688-1.
- ↑ 29.0 29.1 Kozikowski GR. (1996). "Foray Report from Skye". Mycologist 10: 183–84. doi:10.1016/S0269-915X(96)80022-X.
- ↑ 30.0 30.1 Grund DW, Harrison AK. (1976). Nova Scotian Boletes. Lehre, Germany: J. Cramer. pp. 73–75. ISBN 3768210626.
- ↑ 31.0 31.1 Tylukti EE. (1987). Mushrooms of Idaho and the Pacific Northwest. Vol. 2. Non-gilled Hymenomycetes. Moscow, Idaho: The University of Idaho Press. pp. 9–10. ISBN 0-89301-097-9.
- ↑ 32.0 32.1 32.2 32.3 32.4 Wang Y, Sinclair L, Hall IR, Cole ALJ. (1995). "Boletus edulis sensu lato: a new record for New Zealand". New Zealand Journal of Crop and Horticultural Science 23: 227–31. doi:10.1080/01140671.1995.9513892. http://www.royalsociety.org.nz/includes/download.aspx?ID=89662. Retrieved 2009-12-06.
- ↑ Alexopoulos CJ, Mims CW, Blackwell M. (1996). Introductory Mycology. New York, New York: John Wiley and Sons. p. 495. ISBN 0471522295.
- ↑ Vizzini A, Mello A, Ghignone S, Sechi C, Ruiu P, Bonfante P. (2008). "Boletus edulis complex: from phylogenetic relationships to specific primers". Pagine di Micologia (30): 49–52. ISSN 1122-8911.
- ↑ Beugelsdijk DCM, van der Linde S, Zuccarello GC, den Bakker, Draisma SGA, Noordeloos ME. (2008). "A phylogenetic study of Boletus section Boletus in Europe". Persoonia 20 (20): 1–7. doi:10.3767/003158508X283692. PMID 20467482.
- ↑ Mello A, Ghignone S, Vizzini A, Sechi C, Ruiu P, Bonfante P. (2006). "ITS primers for the identification of marketable boletes". Journal of Biotechnology 121 (3): 318–29. doi:10.1016/j.jbiotec.2005.08.022. PMID 16213623.
- ↑ Lian B, Zang J-P, Hou W-G, Yuan S, Smith DL. (2008). "PCR-based sensitive detection of the edible fungus Boletus edulis from rDNA ITS sequences". Electronic Journal of Biotechnology 11 (3): 1–8. doi:10.2225/vol11-issue3-fulltext-4. ISSN 0717-3458.
- ↑ Volk T. (2004-08). "Boletus barrowsii, Chuck Barrows' bolete". Tom Volk's Fungus of the Month. Department of Biology, University of Wisconsin-La Crosse. http://botit.botany.wisc.edu/Toms_fungi/aug2004.html. Retrieved 2008-09-23.
- ↑ Miller HR, Miller OK. (2006). North American Mushrooms: a Field Guide to Edible and Inedible Fungi. Guilford, Conn: Falcon Guide. p. 392. ISBN 0-7627-3109-5. http://books.google.com/?id=zjvXkLpqsEgC&lpg=PA381&dq=boletus%20edulis&pg=PA392#v=onepage&q=boletus%20edulis. Retrieved 2009-11-04.
- ↑ Evenson VS. (1997). Mushrooms of Colorado and the Southern Rocky Mountains. Boulder, Colorado: Westcliffe Publishers. p. 157. ISBN 978-1565791923. http://books.google.com/?id=EAeDeyqZLq0C&lpg=PA156&dq=boletus%20edulis&pg=PA157#v=onepage&q=boletus%20edulis. Retrieved 2009-10-20.
- ↑ Arora D. (2008). "California porcini: three new taxa, observations on their harvest, and the tragedy of no commons" (PDF). Economic Botany 62 (3): 356–75. doi:10.1007/s12231-008-9050-7 (inactive 2009-11-06). http://www.davidarora.com/uploads/arora_california_porcini.pdf.
- ↑ Wood M, Stevens F. "California Fungi: Boletus regineus". MykoWeb. http://www.mykoweb.com/CAF/species/Boletus_regineus.html. Retrieved 2009-11-02.
- ↑ Wood M, Stevens F. "California Fungi: Boletus rex-veris". MykoWeb. http://www.mykoweb.com/CAF/species/Boletus_rex-veris.html. Retrieved 2009-11-03.
- ↑ 44.0 44.1 44.2 44.3 44.4 44.5 44.6 Hall IR, Lyon AJE, Wang Y, Sinclair L. (1998). "Ectomycorrhizal fungi with edible fruiting bodies 2. Boletus edulis". Economic Botany 52 (1): 44–56.
- ↑ 45.0 45.1 45.2 Hall IR, Stephenson SR, Buchanan PK, Yun W, Cole A. (2003). Edible and Poisonous Mushrooms of the World. Portland, Oregon: Timber Press. pp. 224–25. ISBN 0881925861.
- ↑ Oria-de-Rueda J, Martin-Pinto P, Olaizola J. (2008). "Bolete productivity of cistaceous scrublands in northwestern Spain". Economic Botany 62 (3): 323–30. doi:10.1007/s12231-008-9031-x (inactive 2009-11-06).
- ↑ Giri A, Rana P. (2007). "Some Higher Fungi from Sagarmatha National Park (SNP) and its adjoining areas, Nepal". Scientific World 5 (5): 67–74.
- ↑ Adhikary RK, Baruah P, Kalita P, Bordoloi D. (1999). "Edible mushrooms growing in forests of Arunachal Pradesh". Advances in Horticulture and Forestry 6: 119–23.
- ↑ Orlovich D, Stringer A, Yun W, Hall I, Prime G, Danell E, Weden C, Bulman S. (2004). "Boletus edulis Bull. Ex Fries in New Zealand". Australasian Mycological Society Newsletter 1 (1): 6.
- ↑ Eicker A. (1990). "Commercial mushroom production in South Africa". Bulletin (Pretoria: Department of Agricultural Development) (418).
- ↑ Marais LJ, Kotzé JM. (1977). "Notes on ectotrophic mycorrhizae of Pinus patula in South Africa". South African Forestry Journal 100: 61–71.
- ↑ 52.0 52.1 Masuka AJ. (1996). "Dynamics of mushroom (Boletus edulis) production in pine plantations in Zimbabwe". JASSA, Journal of Applied Science in Southern Africa 2 (2): 69–76.
- ↑ Nilson S, Persson O. (1977). Fungi of Northern Europe 1: Larger Fungi (Excluding Gill-Fungi). Harmondsworth, England: Penguin. p. 100. ISBN 0-14-063005-8.
- ↑ Salerni E, Perini C. (2004). "Experimental study for increasing productivity of Boletus edulis s.l. in Italy". Forest Ecology and Management 201 (2–3): 161–70. doi:10.1016/j.foreco.2004.06.027.
- ↑ Baar J, Ter Braak CJF. (1996). "Ectomycorrhizal sporocarp occurrence as affected by manipulation of litter and humus layers in Scots pine stands of different age". Applied Soil Ecology 4 (1): 61–73. doi:10.1016/0929-1393(96)00097-2.
- ↑ Baar J, de Vries FW. (1995). "Effects of manipulation of litter and humus layer on ectomycorrhizal colonization potential in Scots pine stands of different age" (First page preview PDF). Mycorrhiza 5 (4): 267–72. doi:10.1007/BF00204960. http://www.springerlink.com/content/k505pr50v0732860/fulltext.pdf?page=1.
- ↑ Kasparavicius, J. (2001). "Influence of climatic conditions on the growth of fruit bodies of Boletus edulis". Botanica Lithuanica 7 (1): 73–78. ISSN 1392-1665.
- ↑ Martín-Pinto P, Vaquerizo H, Peñalver F, Olaizola J, Oria-de-Rueda J. (2006). "Early effects of a wildfire on the diversity and production of fungal communities in Mediterranean vegetation types dominated by Cistus ladanifer and Pinus pinaster in Spain". Forest Ecology and Management 225 (1–3): 296–305. doi:10.1016/j.foreco.2006.01.006.
- ↑ Quan L, Lei Z-P. (2000). "A study on effect of ectomycorrhizae on promoting Castanea mollissima resistance to drought and its mechanism" (in Chinese). Forest Research 13 (3): 249–56. ISSN 1001-1498.
- ↑ Fu S-C, Tan Q, Chen MJ, Shang X-D, Cai LY, Zhang M-Y. (2009). "Mycorrhizal synthesis involving Boletus edulis and Pinus massoniana". Acta Edulis Fungi 16 (1): 31–41. ISSN 1005-9873.
- ↑ Gross E, Thomazini-Casagrande LI, Caetano FH. (1998). "A scanning electron microscopy study of ectomycorrhizae on Pinus patula Schiede and Deppe". Naturalia (Rio Claro) 23 (1): 93–101. ISSN 0101-1944.
- ↑ Ceruti A, Tozzi M, Reitano G. (1987/88). "Mycorrhizal synthesis between Boletus edulis, Pinus sylvestris and Picea excelsa" (in Italian). Allionia (Turin) 28: 117–24. ISSN 0065-6429.
- ↑ Gobl F. (1977). "Mycorrhiza in Austrian Douglas fir stands" (in German). Centralblatt fur das Gesamte Forstwesen 94 (4): 185–94. ISSN 0379-5292.
- ↑ Froidevaux L, Amiet R. (1975). "Ecto mycorrhizae endo mycorrhizae of Pinus mugo plus Boletus edulis ssp edulis and Pinus cembra plus Suillus variegatus formed in pure culture". European Journal of Forest Pathology 5 (1): 57–61. ISSN 0300-1237.
- ↑ Vozzo JA, Hackskaylo E. (1961). "Mycorrhizal fungi on Pinus virginiana". Mycologia 53 (5): 538–39. doi:10.2307/3756310. http://jstor.org/stable/3756310.
- ↑ 66.0 66.1 Agueda B, Parlade J, Fernandez-Toiran LM, Cisneros O, de Miguel AM, Modrego MP, Martinez-Pena F, Pera J. (2008). "Mycorrhizal synthesis between Boletus edulis species complex and rockroses (Cistus sp.)". Mycorrhiza 18 (8): 443–49. doi:10.1007/s00572-008-0192-3. PMID 18695982.
- ↑ Águeda B, Parladé J, de Miguel AM, Martínez-Peña F. (2006). "Characterization and identification of field ectomycorrhizae of Boletus edulis and Cistus ladanifer". Mycologia 98 (1): 23–30. doi:10.3852/mycologia.98.1.23. PMID 16800301. http://www.mycologia.org/cgi/content/full/98/1/23. Retrieved 2009-11-23.
- ↑ Hall IR, Wang Y, Amicucci A. (2003). "Cultivation of edible ectomycorrhizal mushrooms". Trends in Biotechnology 21 (10): 433–38. doi:10.1016/S0167-7799(03)00204-X. PMID 14512229.
- ↑ Peinter U, Iotti M, Klotz P, Bonuso E, Zambonelli A. (2007). "Soil fungal communities in a Castanea sativa (chestnut) forest producing large quantities of Boletus edulis sensu lato (porcini): where is the mycelium of porcini?". Environmental Microbiology 9 (4): 880–89. doi:10.1111/j.1462-2920.2006.01208.x. PMID 17359260.
- ↑ Collin-Hansen C, Pedersen SA, Andersen RA, Steinnes E. (2007). "First report of phytochelatins in a mushroom: induction of phytochelatins by metal exposure in Boletus edulis". Mycologia 99 (2): 161–74. doi:10.3852/mycologia.99.2.161. PMID 17682769.
- ↑ Kuo M. "Hypomyces chrysospermus". MushroomExpert.Com. http://www.mushroomexpert.com/hypomyces_chrysospermus.html. Retrieved 2009-11-02.
- ↑ Lunghini D, Onofri S, Zucconi L. (1984). "Some cases of intoxication probably caused by Sepedonium spp. infecting fruiting-bodies of some species of Boletus" (in Italian). Micologia Italiana 13 (1): 1/37–1/38. ISSN 0390-0460.
- ↑ Bruns TD. (1984). "Insect mycophagy in the Boletales: fungivore diversity and the mushroom habitat". In Wheeler Q, Blackwell M. Fungus-Insect Relationships: Perspectives in Ecology and Evolution. New York, NY: Columbia University Press. pp. 91–129. ISBN 978-0231056953.
- ↑ Huttinga H, Wichers HJ, Dieleman van Zaayen A. (1975). "Filamentous and polyhedral virus-like particle in Boletus edulis" (PDF). Netherlands Journal of Plant Pathology 81: 102–106. doi:10.1007/BF01999860. http://www.springerlink.com/content/t104136x1388073k/fulltext.pdf?page=1. Retrieved 2009-11-02.
- ↑ Pisi A, Bellardi M, G, Bernicchia A. (1988). "Virus-like particles in Boletus edulis Bull. ex Fr. in Italy" (PDF). Phytopathologia Mediterranea 27 (2): 115–18. ISSN 0031-9465. http://www.google.ca/url?sa=t&source=web&ct=res&cd=1&ved=0CAwQFjAA&url=http%3A%2F%2Fwww.mycologia.org%2Fcgi%2Freprint%2F94%2F5%2F757.pdf&rct=j&q=Feeding+activities+of+slugs+on+myxomycetes+and+macrofungi&ei=57_vSpq2IY6EMtzcsZYH&usg=AFQjCNHz3UDQcrXv0lSb8XgfiLNcUeNetA. Retrieved 2009-11-03.
- ↑ Keller HW, Snell KL. (2002). "Feeding activities of slugs on Myxomycetes and macrofungi". Mycologia 94 (5): 757–60. doi:10.2307/3761690. http://jstor.org/stable/3761690.
- ↑ Bozinovic F, Muñoz-Pedreros A. (1995). "Nutritional ecology and digestive responses of an omnivorous-insectivorous rodent (Abrothrix longipilis) feeding on fungus". Physiological Zoology 68 (3): 474–89.
- ↑ Grönwall O, Pehrson Å. (1984). "Nutrient content in fungi as a primary food of the Red Squirrel Sciurus vulgaris L.". Oecologia 64 (2): 230–31. doi:10.1007/BF00376875.
- ↑ Schiller AM, Larson KW. (2006). "Fox Sparrow foraging on a king bolete mushroom". Northwestern Naturalist 87 (3): 252. doi:10.1898/1051-1733(2006)87[252:FSFOAK]2.0.CO;2.
- ↑ Morford M. (1977). "Juvenal's Fifth Satire". The American Journal of Philology 98 (3): 219–45. doi:10.2307/293772. http://www.jstor.org/pss/293772. Retrieved 2009-12-06.
- ↑ Brothwell DR, Brothwell P. (1998) [1969]. Food in Antiquity: a Survey of the Diet of Early Peoples. Baltimore, MD: The Johns Hopkins University Press. p. 92. ISBN 0801857406.
- ↑ Rolfe RT, Rolfe EW. (1974). The Romance of the Fungus World. New York: Dover. p. 287. ISBN 0-486-23105-4.
- ↑ 83.0 83.1 Jaworska G, Bernaś E. (2008). "The effect of preliminary processing and period of storage on the quality of frozen Boletus edulis (Bull: Fr.) mushrooms". Food Chemistry 113 (4): 936–43. doi:10.1016/j.foodchem.2008.08.023.
- ↑ Melgar MJ, Alonson J, Garcia MA. (2009). "Mercury in edible mushrooms and underlying soil: Bioconcentration factors and toxicological risk". Science of the Total Environment 407 (1): 5328–34. doi:10.1016/j.scitotenv.2009.07.001. PMID 19631362.
- ↑ Collin-Hansen C, Yttri KE, Andersen RA, Berthelsen BO, Steinnes E. (2002). "Mushrooms from two metal-contaminated areas in Norway: occurrence of metals and metallothionein-like proteins". Geochemistry: Exploration, Environment, Analysis 2 (2): 121–30. doi:10.1144/1467-787302-015.
- ↑ Collin-Hansen C, Andersen RA, Steinnes E. (2005). "Molecular defense systems are expressed in the king bolete (Boletus edulis) growing near metal smelters". Mycologia 97 (5): 973–83. doi:10.3852/mycologia.97.5.973. PMID 16596949.
- ↑ Collin-Hansen C, Andersen RA, Steinnes E. (2005). "Damage to DNA and lipids in Boletus edulis exposed to heavy metals". Mycological Research 109 (12): 1386–96. doi:10.1017/S0953756205004016. PMID 16353638.
- ↑ Marzano FN, Bracchi PG, Pizzetti P. (2001). "Radioactive and conventional pollutants accumulated by edible mushrooms (Boletus sp.) are useful indicators of species origin". Environmental Research 85 (3): 260–64. doi:10.1006/enrs.2001.4233. PMID 11237515.
- ↑ Grodzinskaya AA, Berreck M, Haselwandter K, Wasser SP. (2003). "Radiocesium contamination of wild-growing medicinal mushrooms in Ukraine". International Journal of Medicinal Mushrooms 5 (1): 61–86. doi:10.1615/InterJMedicMush.v5.i1.90. ISSN 1521-9437.
- ↑ 90.0 90.1 Lamaison J-L, Polese J-M. (2005). The Great Encyclopedia of Mushrooms. Koln, Germany: Könemann. p. 28. ISBN 3-8331-1239-5.
- ↑ Ramsbottom J. (1947). "Some edible fungi". The British Medical Journal 2 (4250): 304–305. doi:10.1136/bmj.2.4520.304.
- ↑ Carluccio, p. 166.
- ↑ Olney R. (1995). A Provençal Table. London: Pavilion. pp. 31–32. ISBN 1-85793-632-9.
- ↑ Philpot R. (1965). Viennese Cookery. London: Hodder & Stoughton. pp. 139–40.
- ↑ Carluccio, p. 99.
- ↑ Carluccio, p. 96.
- ↑ 97.0 97.1 Arora D. (1986). Mushrooms Demystified: A Comprehensive Guide to the Fleshy Fungi. Berkeley, California: Ten Speed Press. p. 30. ISBN 0898151694.
- ↑ 98.0 98.1 Carluccio, p. 97.
- ↑ Gates L, Delong D. (1992). How to Dry Foods. Los Angeles, California: HP Books. p. 61. ISBN 1-55788-050-6. http://books.google.com/?id=aSBfsPQP_MQC&lpg=PA61&dq=cooking%20Boletus%20edulis&pg=PA61#v=onepage&q=cooking%20Boletus%20edulis. Retrieved 2009-10-06.
- ↑ Seed D. (1987). The Top One Hundred Pasta Sauces. Berkeley, California: Ten Speed Press. p. 25. ISBN 0-89815-232-1. http://books.google.com/?id=5JfdNVZlwA0C&lpg=PA25&dq=cooking%20Boletus%20edulis&pg=PA25#v=onepage&q=cooking%20Boletus%20edulis. Retrieved 2010-11-06.
- ↑ Misharina TA, Muhutdinova SM, Zharikova GG, Terenina MB, Krikunova NI. (2009). "The composition of volatile components of cepe (Boletus edulis) and oyster mushrooms (Pleurotus ostreatus)". Applied Biochemistry and Microbiology 45 (2): 187–93. doi:10.1134/S0003683809020124.
- ↑ Sitta N, Floriani M. (2008). "Nationalization and globalization trends in the wild mushroom commerce of Italy with emphasis on porcini (Boletus edulis and allied species)". Economic Botany 62 (3): 307–22. doi:10.1007/s12231-008-9037-4 (inactive 2009-11-06).
- ↑ 103.0 103.1 103.2 Boa E. (2004). Wild Edible Fungi: A Global Overview of Their Use and Importance to People (Non-Wood Forest Products). Rome, Italy: Food & Agriculture Organization of the UN. pp. 96–97. ISBN 92-5-105157-7. http://books.google.com/?id=Zd2NlcNZgvcC&lpg=PA97&dq=boletus%20edulis&pg=PA97#v=onepage&q=boletus%20edulis. Retrieved 2009-10-30.
- ↑ Sitta N. (2000). "Presence of Tylopilus into dried "Porcini" mushrooms from China" (in Italian). Micologia Italiana 29 (1): 96–99. ISSN 0390-0460.
- ↑ Focus Information Agency, Bulgaria. (in Bulgarian) "По 1 тон гъби на ден се предават в изкупвателните пунктове в Белоградчик [One ton of mushrooms a day are being submitted to purchasing stations in Belogradchik]". Belogradchik.info. http://www.belogradchik.info/1/content/view/390/2/lang,bg/ (in Bulgarian). Retrieved 2009-11-02.
- ↑ Drumeva M, Gyosheva M. "The Macromycetes Fungi of Bulgaria". World Wildlife Fund. http://www.worldwildlife.org/bsp/publications/europe/bulgaria/bulgaria1.html. Retrieved 2009-11-27.
- ↑ Chang S-T, Miles PG. (2004). Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact (2nd ed.). Boca Raton, Florida: CRC Press. p. 131. ISBN 0-8493-1043-1.
- ↑ Veselkov IM. (1975). "Artificial propagation of Boletus edulis in forests". Растительньіе Ресурсы России 11: 574–78.
- ↑ Ceruti A, Tozzi M, Reitano G. (1988). "Micorize di sintesi tra Boletus edulis, Pinus sylvestris e Picea excelsa" (in Italian). Allionia (Turin) 28: 117–24.
- ↑ Fitter AH, Garbaye J. (1994). "Interaction between mycorrhizal fungi and other organisms". In Robson AD, Abbott LK, Malajczuk N. Management of Mycorrhizas in Agriculture, Horticulture and Forestry. Dordrecht, Netherlands: Kluwer Academic Publishers. ISBN 978-0792327004.
- ↑ Nutritional values are based on chemical analysis of Turkish specimens, conducted by Çaglarlrmak and colleagues at the Agricultural Faculty, Food Engineering Department, Gaziosmanpasha University. Source: Çaglarlrmak N, Ünal K, Ötles S. (2001). "Nutritional value of edible wild mushrooms collected from the Black Sea region of Turkey" (PDF). Micologia Aplicada International 14 (1): 1–5. http://www.micaplint.com/PDF/V14_1_01.pdf.
- ↑ 112.0 112.1 Ouzouni PK, Riganakos KA. (2006). "Nutritional value and metal content profile of Greek wild edible fungi". Acta Alimentaria 36 (1): 99–110. doi:10.1556/AAlim.36.2007.1.11.
- ↑ Crisan EV, Sands A (1978). "Nutritional value". In Chang ST, Hayes WA. The Biology and Cultivation of Edible Mushrooms. New York: Academic Press. pp. 727–93. ISBN 978-0121680503.
- ↑ 114.0 114.1 114.2 Kalač P. (2009). "Chemical composition and nutritional value of European species of wild growing mushrooms: a review". Food Chemistry 113 (1): 9–16. doi:10.1016/j.foodchem.2008.07.077.
- ↑ Pedneault K, Angers P, Gosselin A, Tweddell RJ. (2006). "Fatty acid composition of lipids from mushrooms belonging to the family Boletaceae". Mycological Research 110 (Pt 10): 1179–83. doi:10.1016/j.mycres.2006.05.006. PMID 16959482.
- ↑ Ribeiro B, Andrade PB, Silva BM, Baptista P, Seabra RM, Valento P. (2008). "Comparative study on free amino acid composition of wild edible mushroom species". Journal of Agricultural and Food Chemistry 56 (22): 10973–79. doi:10.1021/jf802076p. PMID 18942845. http://pubs.acs.org/doi/abs/10.1021/jf802076p.
- ↑ Tsai S-Y, Tsai H-L, Mau J-L. (2008). "Non-volatile taste components of Agaricus blazei, Agrocybe cylindracea and Boletus edulis". Food Chemistry 107 (3): 977–83. doi:10.1016/j.foodchem.2007.07.080.
- ↑ Falandysz J. (2008). "Selenium in edible mushrooms". Journal of Environmental Science and Health Part C–Environmental Carcinogenesis & Ecotoxicology Reviews 26 (3): 256–99. doi:10.1080/10590500802350086. PMID 18781538.
- ↑ Mutanen M. (1986). "Bioavailability of selenium in mushrooms". International Journal for Vitamin and Nutrition Research 56 (3): 297–301. PMID 3781756.
- ↑ 120.0 120.1 Mattila P, Lampi A-M, Ronkainen R, Toivo J, Piironen V. (2002). "Sterol and vitamin D2 contents in some wild and cultivated mushrooms". Food Chemistry 76 (3): 293–98. doi:10.1016/S0308-8146(01)00275-8.
- ↑ Krzyczkowskia W, Malinowskaa E, Suchockib P, Klepsa J, Olejnikd M, Herold F. (2008). "Isolation and quantitative determination of ergosterol peroxide in various edible mushroom species". Food Chemistry 113 (1): 351–55. doi:10.1016/j.foodchem.2008.06.075.
- ↑ Zheng S, Li C, Ng TB, Wang HX. (2007). "A lectin with mitogenic activity from the edible wild mushroom Boletus edulis". Process Biochemistry 42 (12): 1620–24. doi:10.1016/j.procbio.2007.09.004.
- ↑ Kandefer-Szersen M, Kawecki Z, Salata B, Witek M. (1980). "Mushrooms as a source of substances with antiviral activity" (in Polish). Acta Mycologica 16 (2): 215–20. ISSN 0001-625X.
- ↑ Li D, Zhao W-H, Kong B-H, Ye M, Chen H-R. (2009). "Inhibition effects of the extract and polysaccharide in macrofungus on TMV". Journal of Yunnan Agricultural University 24 (2): 175–80. ISSN 1004-390X.
- ↑ Piraino FF. (2006). "Emerging antiviral drugs from medicinal mushrooms". International Journal of Medicinal Mushrooms 8 (2): 101–14. doi:10.1615/IntJMedMushr.v8.i2.20. ISSN 1521-9437.
- ↑ Ribeiroa B, Lopesa R, Andradea PB, Seabraa RM, Gonçalvesa, Baptistab P, Quelhasa I, Valentão P. (2008). "Comparative study of phytochemicals and antioxidant potential of wild edible mushroom caps and stipes". Food Chemistry 110 (1): 47–56. doi:10.1016/j.foodchem.2008.01.054.
- ↑ Ey J, Schömig E, Taubert D. (2007). "Dietary sources and antioxidant effects of ergothioneine". Journal of Agricultural and Food Chemistry 55 (16): 6466–74. doi:10.1021/jf071328f. PMID 17616140.
- ↑ Lucas EH, Montesono R, Pepper MS, Hafner M, Sablon E. (1957). "Tumor inhibitors in Boletus edulis and other holobasidiomycetes". Antibiotics and Chemotherapy 7: 1–4.
Cited texts
- Carluccio A. (2003-10-17). The Complete Mushroom Book. London, England: Quadrille. ISBN 978-1-844-00040-1.
External links
- Boletus edulis in MycoBank.
- B. edulis in Index Fungorum.
- Mushroom-Collecting.com King bolete
