Vitamin D


Vitamin D is a group of fat-soluble secosteroids, the two major physiologically relevant forms of which are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D without a subscript refers to either D2 or D3 or both. Vitamin D3 is produced in the skin of vertebrates after exposure to ultraviolet B light from the sun or artificial sources, and occurs naturally in a small range of foods. In some countries staples such as milk, flour and margarine are artificially fortified with vitamin D, and it is also available as a supplement in pill form.[2] Food sources such as fatty fish, eggs, and meat are rich in vitamin D and are often recommended for consumption to those suffering vitamin D deficiency.[3]
Vitamin D is carried in the bloodstream to the liver, where it is converted into the prohormone calcidiol. Circulating calcidiol may then be converted into calcitriol, the biologically active form of vitamin D, either in the kidneys or by monocyte-macrophages in the immune system. When synthesized by monocyte-macrophages, calcitriol acts locally as a cytokine, defending the body against microbial invaders.[4]
When synthesized in the kidneys, calcitriol circulates as a hormone, regulating, among other things, the concentration of calcium and phosphate in the bloodstream, promoting the healthy mineralization, growth and remodeling of bone, and the prevention of hypocalcemic tetany. Vitamin D insufficiency can result in thin, brittle, or misshapen bones, while sufficiency prevents rickets in children and osteomalacia in adults, and, together with calcium, helps to protect older adults from osteoporosis. Vitamin D also modulates neuromuscular function, reduces inflammation, and influences the action of many genes that regulate the proliferation, differentiation and apoptosis of cells.[5]
Contents |
Forms
| Name | Chemical composition | Structure |
|---|---|---|
| Vitamin D1 | molecular compound of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) | 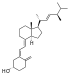 |
| Vitamin D3 | cholecalciferol (made from 7-dehydrocholesterol in the skin). | 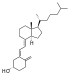 |
| Vitamin D4 | 22-dihydroergocalciferol | 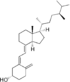 |
| Vitamin D5 | sitocalciferol (made from 7-dehydrositosterol) | 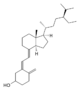 |
Several forms (vitamers) of vitamin D have been discovered (see table). The two major forms are vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol. These are known collectively as calciferol.[6] Vitamin D2 was chemically characterized in 1932. In 1936 the chemical structure of vitamin D3 was established and resulted from the ultraviolet irradiation of 7-dehydrocholesterol.[7]
Chemically, the various forms of vitamin D are secosteroids; i.e., steroids in which one of the bonds in the steroid rings is broken.[8] The structural difference between vitamin D2 and vitamin D3 is in their side chains. The side chain of D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24.
Vitamin D2 (made from ergosterol) is produced by invertebrates, fungus and plants in response to UV irradiation; it is not produced by vertebrates.[9] Little is known about the biologic function of vitamin D2 in nonvertebrate species. Because ergosterol can more efficiently absorb the ultraviolet radiation that can damage DNA, RNA and protein it has been suggested that ergosterol serves as a sunscreening system that protects organisms from damaging high energy ultraviolet radiation.[10]
Vitamin D3 is made in the skin when 7-dehydrocholesterol reacts with UVB ultraviolet light at wavelengths between 270–300 nm, with peak synthesis occurring between 295-297 nm.[11] These wavelengths are present in sunlight when the UV index is greater than 3. At this solar elevation, which occurs daily within the tropics, daily during the spring and summer seasons in temperate regions, and almost never within the arctic circles, vitamin D3 can be made in the skin. Depending on the intensity of UVB rays and the minutes of exposure, an equilibrium can develop in the skin, and vitamin D degrades as fast as it is generated.[12]
Evolution
The photosynthesis of vitamin D evolved over 750 million years ago; the phytoplankton coccolithophor Emeliani huxleii is an early example. Vitamin D played a critical role in the maintenance of a calcified skeleton in vertebrates as they left their calcium-rich ocean environment for land over 350 million years ago. "Because vitamin D can only be synthesized via a photochemical process, early vertebrates that ventured onto land either had to ingest foods that contained vitamin D or had to be exposed to sunlight to photosynthesize vitamin D in their skin to satisfy their body’s vitamin D requirement."[12]
Production in the skin

Vitamin D3 is made in the skin when 7-dehydrocholesterol absorbs UVB ultraviolet light at wavelengths between 270–300 nm, with peak synthesis occurring between 295-297 nm.[11]
The skin consists of two primary layers: the inner layer called the dermis, composed largely of connective tissue, and the outer, thinner epidermis. The epidermis consists of five strata; from outer to inner they are: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. Vitamin D is produced in the two innermost strata, the stratum basale and stratum spinosum.
Cholecalciferol is produced photochemically in the skin from 7-dehydrocholesterol; 7-dehydrocholesterol is produced in relatively large quantities in the skin of most vertebrate animals, including humans.[13] The naked mole rat appears to be naturally cholecalciferol deficient as serum 25-OH vitamin D levels are undetectable.[14] Interestingly, the naked mole rat is resistant to aging, maintains healthy vascular function[15] and is the longest lived of all rodents.[16]
In some animals, the presence of fur or feathers blocks the UV rays from reaching the skin. In birds and fur-bearing mammals, vitamin D is generated from the oily secretions of the skin deposited onto the fur and obtained orally during grooming.[17]
In 1923, it was established that when 7-dehydrocholesterol is irradiated with light, a form of a fat-soluble vitamin is produced. Alfred Fabian Hess showed that "light equals vitamin D".[18] Adolf Windaus, at the University of Göttingen in Germany, received the Nobel Prize in Chemistry in 1928, for his work on the constitution of sterols and their connection with vitamins.[19] In the 1930s he clarified further the chemical structure of vitamin D.
In 1923, Harry Steenbock at the University of Wisconsin demonstrated that irradiation by ultraviolet light increased the vitamin D content of foods and other organic materials.[20] After irradiating rodent food, Steenbock discovered that the rodents were cured of rickets. It is now known that vitamin D deficiency is a cause of rickets. Using $300 of his own money, Steenbock patented his invention. Steenbock's irradiation technique was used for foodstuffs, most memorably for milk. By the expiration of his patent in 1945, rickets had all but been eliminated in the US.[21]
Synthesis mechanism (form 3)
| In the skin 7-dehydrocholesterol, a derivative of cholesterol, is photolyzed by ultraviolet light in a 6-electron conrotatory electrocyclic reaction. The product is pre-vitamin D3. | 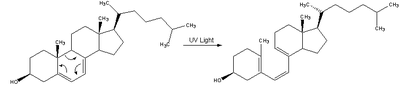 |
| Previtamin D3 spontaneously isomerizes to vitamin D3 (cholecalciferol) in a antarafacial sigmatropic [1,7] hydride shift. At room temperature the transformation of previtamin D3 to vitamin D3 takes about 12 days to complete.[10] | 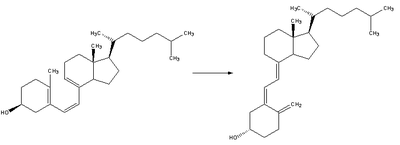 |
| Whether it is made in the skin or ingested, vitamin D3 (cholecalciferol) is hydroxylated in the liver at position 25 (upper right of the molecule) forming 25-hydroxycholecalciferol (calcidiol). This reaction is catalyzed by the microsomal enzyme vitamin D 25-hydroxylase,[22] which is produced by hepatocytes. Once made the product is stored in the hepatocytes until it is needed and then can be released into the plasma where it will be bound to an α-globulin. | 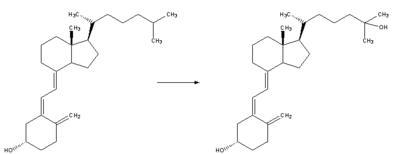 |
| Calcidiol is transported to the proximal tubules of the kidneys where it is hydroxylated at the 1-α position (lower right of the molecule) forming calcitriol. This product is a potent ligand of the vitamin D receptor (VDR) which mediates most of the physiological actions of the vitamin. The conversion of calcidiol to calcitriol is catalyzed by the enzyme 25-hydroxyvitamin D3 1-alpha-hydroxylase, the levels of which are increased by parathyroid hormone (and additionally by low calcium or phosphate). | 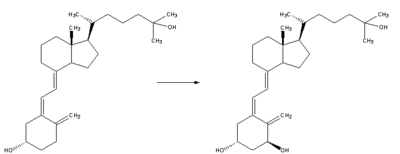 |
Mechanism of action
Following the final converting step in the kidney, calcitriol (the physiologically active form of vitamin D) is released into the circulation. By binding to vitamin D-binding protein (VDBP), a carrier protein in the plasma, calcitriol is transported to various target organs.[8]
Calcitriol mediates its biological effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells.[8] The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which are involved in calcium absorption in the intestine.
The vitamin D receptor belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDRs are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate, and breast. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content.[23]
The VDR is known to be involved in cell proliferation and differentiation. Vitamin D also affects the immune system, and VDRs are expressed in several white blood cells, including monocytes and activated T and B cells.[24]
Apart from VDR activation, various alternative mechanisms of action are known. An important one of these is its role as a natural inhibitor of signal transduction by hedgehog (a hormone involved in morphogenesis).[25][26]
Nutrition

Adequate intake
Adequate intake levels of vitamin D have been established by the Food and Nutrition Board at the Institute of Medicine of The National Academies (formerly National Academy of Sciences). Adequate intakes depend only on age (i.e., they are the same regardless of sex, pregnancy, or lactation).
- Birth to 50 years, 5 µg (200 IU)
- 51–70 years, 10 µg (400 IU)
- 71+ years, 15 µg (600 IU)
These intake levels are based on the assumption that the vitamin is not synthesized by exposure to sunlight.
In the United States, typical diets provide about 100 IU/day. The NIH has a "Tolerable Upper Intake Level (UL)" of 2,000 IU/day, while newer studies indicate a UL as high as 10,000 IU/day.[27]
Australian studies into vitamin D deficiency have yielded tables of recommended sunlight intake based on the country's major cities.[3]
Natural sources

Natural sources of vitamin D include:[5]
- Fatty fish species, such as:
- Catfish, 85 g (3 oz) provides 425 IU (5 IU/g)
- Salmon, cooked, 100 g (3.5 oz) provides 360 IU (3.6 IU/g)
- Mackerel, cooked, 100 g (3.5 oz), 345 IU (3.45 IU/g)
- Sardines, canned in oil, drained, 50 g (1.75 oz), 250 IU (5 IU/g)
- Tuna, canned in oil, 100 g (3.5 oz), 235 IU (2.35 IU/g)
- Eel, cooked, 100 g (3.5 oz), 200 IU (2.00 IU/g)
- A whole egg, provides 20 IU (0.33 IU/g if egg weighs 60 g)
- Beef liver, cooked, 100 g (3.5 oz), provides 15 IU (0.15 IU/g)
- Fish liver oils, such as cod liver oil, 1 Tbs. (15 ml) provides 1360 IU (90.6 IU/ml)
- Mushrooms are the only vegan source of vitamin D (besides UV light or sunlight exposure).[28][29] 100g provides: (regular) 14 IU (0.14 IU/g), (exposed to UV) 500 IU (5 IU/g)[30]
Nutrition Facts labels on food products in the US are not required to list vitamin D content unless a food has been fortified with this nutrient.[5]
Naming of vitamin D
In 1913 American researchers Elmer McCollum and Marguerite Davis discovered a substance in cod liver oil which later came to be called "vitamin A". A British doctor Edward Mellanby noticed that dogs that were fed cod liver oil did not develop rickets and concluded that vitamin A, or a closely associated factor, could prevent the disease. In 1921 Elmer McCollum tested modified cod liver oil in which the vitamin A had been destroyed. The modified oil cured the sick dogs, so McCollum concluded that the factor in cod liver oil which cured rickets was distinct from vitamin A. He called it vitamin D because it was the fourth vitamin to be named.[31][32][33] Unlike other vitamins, vitamin D can be synthesised by humans and it is therefore not a vitamin in the strict sense.
Measuring vitamin D status
The serum concentration of 25-hydroxy-vitamin D is typically used to determine vitamin D status. It reflects vitamin D produced in the skin as well as that acquired from the diet, and has a fairly long circulating half-life of 15 days. It does not, however, reveal the amount of vitamin D stored in other body tissues. The level of serum 1,25-dihydroxy-vitamin D is not usually used to determine vitamin D status because it has a short half-life of 15 hours and is tightly regulated by parathyroid hormone, calcium, and phosphate, such that it does not decrease significantly until vitamin D deficiency is already well advanced.[5]
There has been variability in results of laboratory analyses of the level of 25-hydroxy-vitamin D. Falsely low or high values have been obtained depending on the particular test or laboratory used. Beginning in July 2009 a standard reference material became available which should allow laboratories to standardise their procedures.[5]
There is some disagreement concerning the exact levels of 25-hydroxy-vitamin D needed for good health. A level lower than 10 ng/mL (25 nmol/L) is associated with the most severe deficiency diseases: rickets in infants and children, and osteomalacia in adults. A concentration above 15 ng/ml (37.5 nmol/L) is generally considered adequate for those in good health. Levels above 30 ng/ml (75 nmol/L) are proposed by some as desirable for achieving optimum health, but there is not yet enough evidence to support this.[5][34][35]
Levels of 25-hydroxy-vitamin D that are consistently above 200 ng/mL (500 nmol/L) are thought to be potentially toxic, although data from humans is sparse. In animal studies levels up to 400 ng/mL (1,000 nmol/L) were not associated with toxicity.[5] Vitamin D toxicity usually results from taking supplements in excess. Hypercalcemia is typically the cause of symptoms, and levels of 25-hydroxy-vitamin D above 150 ng/mL (375 nmol/L) are usually found, although in some cases 25-hydroxy-vitamin D levels may appear to be normal. It is recommended to periodically measure serum calcium in individuals receiving large doses of vitamin D.[36]
In overweight persons increased fat mass is inversely associated with 25(OH)D levels.[37][38] This association may confound the reported relationships between low vitamin D status and conditions which occur more commonly in obesity[39] as the circulating 25(OH)D underestimates their total body stores.[40]
A study of highly sun exposed (tanned) healthy young skateboarders and surfers in Hawaii found levels below the proposed higher minimum of 30 ng/ml in 51% of the subjects. The highest 25(OH)D concentration was around 60 ng/ml (150nmol/L).[41] A similar <using the same data> study in Hawaii found a range of (11–71 ng/mL) in a population with prolonged extensive skin exposure while as part of the same study Wisconsin breastfeeding mothers were given supplements. The range of circulating 25(OH)D levels in women in the supplementated group was from 12–77 ng/mL. It is noteworthy that the levels in the supplemented population in Wisconsin were higher than the sun exposed group in Hawaii (which again included surfers because it was the same data set).[42]
Deficiency
Low blood calcidiol (25-hydroxy-vitamin D) can result from avoiding the sun.[43] Deficiency results in impaired bone mineralization, and leads to bone softening diseases[44] including:
- Rickets, a childhood disease characterized by impeded growth, and deformity, of the long bones which can be caused by calcium or phosphorus deficiency as well as a lack of vitamin D; today it is largely found in low income countries in Africa, Asia or the Middle East[45] and in those with genetic disorders such as pseudovitamin D deficiency rickets.[46] Rickets was first described in 1650 by Francis Glisson who said it had first appeared about 30 years previously in the counties of Dorset and Somerset.[47] In 1857 John Snow suggested the rickets then widespread in Britain was being caused by the adulteration of bakers bread with alum.[48] The role of diet in the development of rickets[49][50] was determined by Edward Mellanby between 1918–1920.[51] Nutritional rickets exists in countries with intense year round sunlight such as Nigeria and can occur without vitamin D deficiency.[52][53] Although rickets and osteomalacia are now rare in Britain there have been outbreaks in some immigrant communities in which osteomalacia sufferers included women with seemingly adequate daylight outdoor exposure wearing Western clothing.[54] Having darker skin and reduced exposure to sunshine did not produce rickets unless the diet deviated from a Western omnivore pattern characterized by high intakes of meat, fish and eggs, and low intakes of high-extraction cereals.[55][56][57] The dietary risk factors for rickets include abstaining from animal foods.[58][59] Vitamin D deficiency remains the main cause of rickets among young infants in most countries, because breast milk is low in vitamin D and social customs and climatic conditions can prevent adequate UVB exposure. In sunny countries such as Nigeria, South Africa, and Bangladesh where the disease occurs among older toddlers and children it has been attributed to low dietary calcium intakes, which are characteristic of cereal-based diets with limited access to dairy products.[57] Rickets was formerly a major public health problem among the US population; in Denver where ultraviolet rays are approximately 20% stronger than at sea level on the same latitude[60] almost two thirds of 500 children had mild rickets in the late 1920s.[61] An increase in the proportion of animal protein[59][62] in the 20th century American diet coupled with increased consumption of milk[63][64] fortified with relatively small quantities of vitamin D coincided with a dramatic decline in the number of rickets cases.[23]
- Osteomalacia, a bone-thinning disorder that occurs exclusively in adults and is characterized by proximal muscle weakness and bone fragility. The effects of osteomalacia are thought to contribute to chronic musculoskeletal pain,[65][66] there is no persuasive evidence of lower vitamin D status in chronic pain sufferers.[67]
Adequate vitamin D may also be associated with healthy hair follicle growth cycles.[68] There are also associations between low 25(OH)D levels and peripheral vascular disease,[69] certain cancers, multiple sclerosis, rheumatoid arthritis, juvenile diabetes,[23] Parkinson's and Alzheimer's disease.[70] However these associations were found in observational studies and vitamin D vitamin supplements have not been demonstrated to reduce the risks of these diseases.[34]
Research shows that dark-skinned people living in Western societies have lower vitamin D levels.[71][72][72] It has been suggested that dark-skinned people are less efficient at making vitamin D because melanin in the skin hinders vitamin D synthesis, however a recent study has found novel evidence that low vitamin D levels among Africans may be due to other reasons.[73] Recent evidence implicates parathyroid hormone in adverse cardiovascular outcomes, black women have an increase in serum PTH at a lower 25(OH)D level than white women.[74] A large scale association study of the genetic determinants of vitamin D insufficiency in Caucasians found no links to pigmentation.[75][76]
Overdose by ingestion
In healthy adults, sustained intake of 1250 micrograms/day (50,000 IU) can produce overt toxicity after several months;[77] those with certain medical conditions are far more sensitive to vitamin D and develop hypercalcaemia in response to any increase in vitamin D nutrition, while maternal hypercalcaemia during pregnancy may increase fetal sensitivity to effects of vitamin D and lead to a syndrome of mental retardation and facial deformities.[78][79] Pregnant or breastfeeding women should consult a doctor before taking a vitamin D supplement. For infants (birth to 12 months) the tolerable Upper Limit (maximum amount that can be tolerated without harm) is set at 25 micrograms/day (1000 IU). 1000 micrograms/day (40,000 IU) in infants has produced toxicity within 1 month.[80] The U.S. Dietary Reference Intake Tolerable Upper Intake Level (upper limit) of vitamin D for children and adults is set at 50 micrograms/day (2,000 IU). Vitamin D overdose causes hypercalcemia and the main symptoms of vitamin D overdose are those of hypercalcemia: anorexia, nausea, and vomiting can occur, frequently followed by polyuria, polydipsia, weakness, nervousness, pruritus, and ultimately renal failure. Proteinuria, urinary casts, azotemia, and metastatic calcification (especially in the kidneys) may develop.[81] Vitamin D toxicity is treated by discontinuing vitamin D supplementation and restricting calcium intake. Kidney damage may be irreversible.
Exposure to sunlight for extended periods of time does not normally cause vitamin D toxicity.[78] This is because within about 20 minutes of ultraviolet exposure in light skinned individuals (3–6 times longer for pigmented skin) the concentrations of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D that is produced is degraded.[12] According to some sources, endogenous production with full body exposure to sunlight is approximately 250 µg (10,000 IU) per day.[78] According to Holick, "the skin has a large capacity to produce cholecalciferol"; his experiments indicate that
"[W]hole-body exposure to one minimal erythemal dose [a dose that would just begin to produce sunburn in a given individual] of simulated solar ultraviolet radiation is comparable with taking an oral dose of between 250 and 625 micrograms (10 000 and 25 000 IU) vitamin D."[12]
The similar effect of supplementation and whole body exposure to one erythemal dose prompted a researcher [82] to suggest that 250 micrograms/day (10,000 IU) in healthy adults should be adopted as the tolerable upper limit.[82] Supplements and skin synthesis have a different effect on serum 25(OH)D concentrations;[83] endogenously synthesized vitamin D3 travels in plasma almost exclusively on vitamin D-binding protein (VDBP), providing for a slower hepatic delivery of the vitamin D and the more sustained increase in plasma 25-hydroxycholecalciferol. Orally administered vitamin D produces swift hepatic delivery and increases in plasma 25-hydroxycholecalciferol. The richest food source of vitamin D — wild salmon — would require 35 ounces a day to provide 10,000IU.[84] Recommending supplementation, when those supposedly in need of it are labeled healthy, has proved contentious, and doubt exists concerning long term effects of attaining and maintaining serum 25(OH)D of at least 80nmol/L by supplementation.[85]
A Toronto study concluded, "skin pigmentation, assessed by measuring skin melanin content, showed an inverse relationship with serum 25(OH)D". The uniform occurrence of low serum 25(OH)D in Indians living in India.[86] and Chinese in China,[87] does not support the hypothesis that the low levels seen in the more pigmented are due to lack of synthesis from the sun at higher latitudes; the leader of the study has urged dark-skinned immigrants to take vitamin D supplements nonetheless, saying, "I see no risk, no downside, there's only a potential benefit".[88][89] Whether the toxicity of oral intake of vitamin D is due to that route being unnatural, as suggested by Fraser,[83] is not known, but there is evidence to suggest that dietary vitamin D may be carried by lipoprotein particles[90] into cells of the artery wall and atherosclerotic plaque, where it may be converted to active form by monocyte-macrophages.[91] These findings raise questions regarding the effects of vitamin D intake on atherosclerotic calcification and cardiovascular risk.
Health effects
Immune system
VDR ligands have been shown to increase the activity of natural killer cells, and enhance the phagocytic activity of macrophages.[24] Active vitamin D hormone also increases the production of cathelicidin, an antimicrobial peptide that is produced in macrophages triggered by bacteria, viruses, and fungi.[92][93][94] Suggestions of a link between vitamin D deficiency and the onset of multiple sclerosis posited that this is due to the immune-response suppression properties of Vitamin D[95] and that vitamin D is required to activate a histocompatibility gene (HLA-DRB1*1501) necessary for differentiating between self and foreign proteins in a subgroup of individuals genetically predisposed to MS.[96] Whether vitamin D supplements during pregnancy can lessen the likelihood of the child developing MS later in life is not known;[97][98] however, vitamin D fortification has been suggested to have caused a pandemic of allergic disease[99] and an association between vitamin D supplementation in infancy and an increased risk of atopy and allergic rhinitis later in life has been found .[100] Veteran vitamin D researcher Hector DeLuca has cast doubt on whether vitamin D affects MS.[101] For viral infections, other implicated factors include low relative humidities produced by indoor heating and cold temperatures that favor virus spread during winter.[102]
Influenza
Lack of vitamin D synthesis is a possible explanation for high rates of influenza infection during winter; however, see flu season for the factors apart from vitamin D that are also hypothesized to influence rates of infection during winter.[103] Other implicated factors include low relative humidities produced by indoor heating and cold temperatures as features of winter that favor influenza virus spread.
Cancer
The molecular basis for thinking vitamin D has the potential to prevent cancer lies in its role in a wide range of cellular mechanisms central to the development of cancer.[104] These effects may be mediated through vitamin D receptors expressed in cancer cells.[24] Polymorphisms of the vitamin D receptor (VDR) gene have been associated with an increased risk of breast cancer.[105] Women with mutations in the VDR gene had an increased risk of breast cancer.[106]
A 2006 study using data on over 4 million cancer patients from 13 different countries showed a marked increase in some cancer risks in countries with less sun and another metastudy found correlations between vitamin D levels and cancer. The authors suggested that intake of an additional 1,000 international units (IU) (or 25 micrograms) of vitamin D daily reduced an individual's colon cancer risk by 50%, and breast and ovarian cancer risks by 30%.[107][108][109][110] Low levels of vitamin D in serum have been correlated with breast cancer disease progression and bone metastases.[105] However, the vitamin D levels of a population do not depend on the solar irradiance to which they are exposed.[111][112][113][114] Moreover, there are genetic factors involved with cancer incidence and mortality which are more common in northern latitudes.[115][116]
A 2006 study found that taking the U.S. RDA of vitamin D (400 IU per day) cut the risk of pancreatic cancer by 43% in a sample of more than 120,000 people from two long-term health surveys.[117][118] However, in male smokers a 3-fold increased risk for pancreatic cancer in the highest compared to lowest quintile of serum 25-hydroxyvitamin D concentration has been found.[119]
A randomized intervention study involving 1,200 women, published in June 2007, reports that vitamin D supplementation (1,100 international units (IU)/day) resulted in a 60% reduction in cancer incidence, during a four-year clinical trial, rising to a 77% reduction for cancers diagnosed after the first year (and therefore excluding those cancers more likely to have originated prior to the vitamin D intervention).[120][121] The study was criticized on several grounds[122] including lack of data, use of statistical techniques and comparison with a self-selected (i.e. non-randomized) observational study that found long term convergence of breast cancer incidence[123] The author's response provided the required data, explained their statistical usage and commented that even if the vitamin D merely delayed the appearance of cancer (which they did not believe, based on other studies), that that was still a considerable benefit.[122]
In 2007 the Canadian Cancer Society, (a national community-based organization of volunteers) recommended that all adults begin taking 1,000 IU per day (five times more than the government says they need) .[124][125] A US National Cancer Institute study analyzed data from the third national Health and Nutrition Examination Survey to examine the relationship between levels of circulating vitamin D in the blood and cancer mortality in a group of 16,818 participants aged 17 and older. It found no support for an association between 25(OH)D and total cancer mortality. However, the study did find that "[c]olorectal cancer mortality was inversely related to serum 25(OH)D level, with levels 80 nmol/L or higher associated with a 72% risk reduction (95% confidence interval = 32% to 89%) compared with lower than 50 nmol/L, Ptrend = .02."[126] Unlike other studies, this one was carried out prospectively — meaning that participants were followed looking forward — and the researchers used actual blood tests to measure the amount of vitamin D in blood, rather than trying to infer vitamin D levels from potentially inaccurate predictive models.[114][127]
Cardiovascular disease
A report from the National Health and Nutrition Examination Survey (NHANES) involving nearly 5,000 participants found that low levels of vitamin D were associated with an increased risk of peripheral artery disease (PAD). The incidence of PAD was 80% higher in participants with the lowest vitamin D levels (<17.8 ng/mL).[69] Cholesterol levels were found to be reduced in gardeners in the UK during the summer months.[128] Low levels of vitamin D are associated with an increase in high blood pressure and cardiovascular risk. Numerous observational studies show this link, but of two systemic reviews one found only weak evidence of benefit from supplements and the other found no evidence of a beneficial effect whatsoever.[129][130][131]
There is a certain amount of evidence to suggest that dietary vitamin D may be carried by lipoprotein particles[90] into cells of the artery wall and atherosclerotic plaque, where it may be converted to active form by monocyte-macrophages.[91] These findings raise questions regarding the effects of vitamin D intake on atherosclerotic calcification and cardiovascular risk. Calcifediol (25-hydroxy-vitamin D) is implicated in the etiology of atherosclerosis, especially in non-Caucasians.[83][85][132][133] Freedman et al. (2010) have found that serum vitamin D correlates in African Americans, but not in Euro-Americans, with calcified atheroscleratic plaque. "Higher levels of 25-hydroxyvitamin D seem to be positively correlated with aorta and carotid CP in African Americans but not with coronary CP. These results contradict what is observed in individuals of European descent".[85][112][113][133][134] One study found an elevated risk of ischaemic heart disease in Southern India in individuals whose vitamin D levels were above 89 ng/mL.[132] A review of vitamin D status in India concluded that studies uniformly point to low 25(OH)D levels in Indians despite abundant sunshine, and suggested a public health need to fortify Indian foods with vitamin D might exist.[135] However the levels found in India are consistent with many other studies of tropical populations which have found that even an extreme amount of sun exposure, such as incurred by rural Indians,[136] does not raise 25(OH)D levels to the levels typically found in Europeans,[111][137][138]
Mortality
Using information from the National Health and Nutrition Examination Survey a large scale study concluded that having low levels of vitamin D (<17.8 ng/ml) was independently associated with an increase in all-cause mortality in the general population.[139] However it has been pointed out that increased mortality was also found in those with higher concentrations, (above 50 ng/ml).[140] A sophisticated August 2010 study of plasma vitamin D and mortality in older men concluded that both high (>39 ng/ml) and low (<18 ng/ml)) concentrations of plasma 25(OH)D are associated with elevated risks of overall and cancer mortality compared with intermediate concentrations. These boundaries were less than suggested by the Melamed et al. study of National Health and Nutrition Examination Survey data[141] but the immunoassay used by National Health and Nutrition Examination Survey tended to overestimate vitamin D values.[142]
Overall, excess or deficiency in the calcipherol system appear to cause abnormal functioning and premature aging.[143][144][145]
Complex regulatory mechanisms control metabolism, recent epidemiologic evidence suggests that there is a narrow range of vitamin D levels in which function is optimized. Levels above or below this natural homeostasis of vitamin D increase mortality.[91][146]
See also
- Mushrooms and vitamin D
- Risks and benefits of sun exposure
- Vitamin D and influenza
References
- ↑ Walter F., PhD. Boron (2003). "The Parathyroid Glands and Vitamin F". Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1094. ISBN 978-1-4160-2328-9.
- ↑ Institute of Medicine (IOM). Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride (1997) Access date: 2010-04-14 [1]
- ↑ 3.0 3.1 Joshi, D; Center, J; Eisman, J (2010). "Vitamin D deficiency in adults". Australian Prescriber 33 (4): 103–6. http://www.australianprescriber.com/magazine/33/4/103/6.
- ↑ Adams, JS; Hewison, M (Feb 2010). "Update in vitamin D". J Clin Endocrinol Metab 95 (2): 471–8. doi:10.1210/jc.2009-1773. PMID 20133466.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 "Dietary Supplement Fact Sheet: Vitamin D". National Institutes of Health Office of Dietary Spplements. http://ods.od.nih.gov/factsheets/vitamind.asp. Retrieved 2010-04-11.
- ↑ Dorland's Illustrated Medical Dictionary, under Vitamin (Table of Vitamins)
- ↑ History of Vitamin D University of California, Riverside, Vitamin D Workshop.
- ↑ 8.0 8.1 8.2 About Vitamin D Including Sections: History, Nutrition, Chemistry, Biochemistry, and Diseases. University of California Riverside
- ↑ [2] Vitamin D - MayoClinic.com
- ↑ 10.0 10.1 Holick, MF (2004). "Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis". The American journal of clinical nutrition 79 (3): 362–71. PMID 14985208.
- ↑ 11.0 11.1 Eleanor Margaret Hume, Nathaniel Sampson Lucas, and Hannah Henderson Smith (1927). "On the Absorption of Vitamin D from the Skin". Biochem J 21 (2): 362–367. PMID PMC1251921. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251921/?page=1.
- ↑ 12.0 12.1 12.2 12.3 Holick MF (March 1995). "Environmental factors that influence the cutaneous production of vitamin D". The American Journal of Clinical Nutrition 61 (3 Suppl): 638S–645S. PMID 7879731. http://www.ajcn.org/cgi/reprint/61/3/638S.
- ↑ Crissey, SD; Ange, KD; Jacobsen, KL; Slifka, KA; Bowen, PE; Stacewicz-Sapuntzakis, M; Langman, CB; Sadler, W et al. (2003). "Serum concentrations of lipids, vitamin d metabolites, retinol, retinyl esters, tocopherols and selected carotenoids in twelve captive wild felid species at four zoos". The Journal of nutrition 133 (1): 160–6. PMID 12514284.
- ↑ Yahav, S; Buffenstein, R (1993). "Cholecalciferol supplementation alters gut function and improves digestibility in an underground inhabitant, the naked mole rat (Heterocephalus glaber), when fed on a carrot diet". The British journal of nutrition 69 (1): 233–41. doi:10.1079/BJN19930025. PMID 8384476.
- ↑ Csiszar, A; Labinskyy, N; Orosz, Z; Xiangmin, Z; Buffenstein, R; Ungvari, Z (2007). "Vascular aging in the longest-living rodent, the naked mole rat". American journal of physiology. Heart and circulatory physiology 293 (2): H919–27. doi:10.1152/ajpheart.01287.2006. PMID 17468332.
- ↑ Buffenstein, R (2008). "Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species". Journal of comparative physiology. B, Biochemical, systemic, and environmental physiology 178 (4): 439–45. doi:10.1007/s00360-007-0237-5. PMID 18180931.
- ↑ Stout, Sam D.; Agarwal, Sabrina C.; Stout, Samuel D. (2003). Bone loss and osteoporosis: an anthropological perspective. New York: Kluwer Academic/Plenum Publishers. ISBN 0-306-47767-X.
- ↑ UNRAVELING THE ENIGMA OF VITAMIN D U.S. National Academy of Sciences
- ↑ "Windaus biography at". Nobelprize.org. 1959-06-09. http://nobelprize.org/nobel_prizes/chemistry/laureates/1928/windaus-bio.html. Retrieved 2010-03-25.
- ↑ Arvids A. Ziedonis; Mowery, David C.; Nelson, Richard R.; Bhaven N. Sampat (2004). Ivory tower and industrial innovation: university-industry technology transfer before and after the Bayh-Dole Act in the United States. Stanford, Calif: Stanford Business Books. pp. 39–40. ISBN 0-8047-4920-5.
- ↑ {Marshall, James (2005). Elbridge A. Stuart Founder of the Carnation Company. Kessinger Publishing. p. 235. ISBN 1417988835
- ↑ Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (18 May 2004). "Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase". Proc Natl Acad Sci U S A 101 (20): 7711–7715. doi:10.1073/pnas.0402490101. PMID 15128933.
- ↑ 23.0 23.1 23.2 Holick, MF (2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". The American journal of clinical nutrition 80 (6 Suppl): 1678S–88S. PMID 15585788.
- ↑ 24.0 24.1 24.2 Vitamin D The Physicians Desk Reference. 2006 Thompson Healthcare.
- ↑ Bijlsma, MF; Spek, CA; Zivkovic, D; Van De Water, S; Rezaee, F; Peppelenbosch, MP (2006). "Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion". PLoS biology 4 (8): e232. doi:10.1371/journal.pbio.0040232. PMID 16895439.
- ↑ "Hedgehog signaling and Vitamin D". Medscape.com. 2009-12-18. http://www.medscape.com/viewarticle/713649_3. Retrieved 2010-03-25.
- ↑ "Dietary Supplement Fact Sheet: Vitamin D". Ods.od.nih.gov. http://ods.od.nih.gov/factsheets/vitamind.asp. Retrieved 2010-03-25.
- ↑ Bowerman, Susan (2008-03-31). "If mushrooms see the light — Los Angeles Times". Articles.latimes.com. http://articles.latimes.com/2008/mar/31/health/he-eat31. Retrieved 2010-03-25.
- ↑ Koyyalamudi, SR; Jeong, SC; Song, CH; Cho, KY; Pang, G (2009). "Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation". Journal of agricultural and food chemistry 57 (8): 3351–5. doi:10.1021/jf803908q. PMID 19281276.
- ↑ "USDA nutrient database - need to search for 11939 and then click submit". http://www.nal.usda.gov/fnic/foodcomp/search/index.html.
- ↑ "Age-old children's disease back in force". Thestar.com. 2007-07-25. http://www.thestar.com/printarticle/239341. Retrieved 2010-08-24.
- ↑ "Los Angeles Times, Fortified foods took out rickets". Articles.latimes.com. 2006-07-24. http://articles.latimes.com/2006/jul/24/health/he-esoterica24. Retrieved 2010-08-24.
- ↑ McClean, Franklin C.; Budy, Ann M. "Vitamin A, Vitamin D, Cartilage, Bones, and Teeth" in Harris, Robert S. (1963). Vitamins and Hormones, volume 21, pp. 51–52. London: Academic Press Partial view at Google Books.
- ↑ 34.0 34.1 Pittas, AG; Chung, M; Trikalinos, T; Mitri, J; Brendel, M; Patel, K; Lichtenstein, AH; Lau, J et al. (2010). "Systematic review: Vitamin D and cardiometabolic outcomes". Annals of internal medicine 152 (5): 307–14. doi:10.1059/0003-4819-152-5-201003020-00009 (inactive 2010-03-25). PMID 20194237.
- ↑ Wang, L; Manson, JE; Song, Y; Sesso, HD (2010). "Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events". Annals of internal medicine 152 (5): 315–23. doi:10.1059/0003-4819-152-5-201003020-00010 (inactive 2010-03-25). PMID 20194238.
- ↑ Vitamin D at The Merck Manual for Healthcare Professionals.
- ↑ Lucas, JA; Bolland, MJ; Grey, AB; Ames, RW; Mason, BH; Horne, AM; Gamble, GD; Reid, IR (2005). "Determinants of vitamin D status in older women living in a subtropical climate.". Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 16 (12): 1641–8. doi:10.1007/s00198-005-1888-2. PMID 16027959.
- ↑ Bolland, MJ; Grey, AB; Ames, RW; Mason, BH; Horne, AM; Gamble, GD; Reid, IR (2006). "Determinants of vitamin D status in older men living in a subtropical climate.". Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 17 (12): 1742–8. doi:10.1007/s00198-006-0190-2. PMID 16932872.
- ↑ Field, AE; Coakley, EH; Must, A; Spadano, JL; Laird, N; Dietz, WH; Rimm, E; Colditz, GA (2001). "Impact of overweight on the risk of developing common chronic diseases during a 10-year period.". Archives of internal medicine 161 (13): 1581–6. doi:10.1001/archinte.161.13.1581. PMID 11434789.
- ↑ Wortsman, J; Matsuoka, LY; Chen, TC; Lu, Z; Holick, MF (2000). "Decreased bioavailability of vitamin D in obesity.". The American journal of clinical nutrition 72 (3): 690–3. PMID 10966885.
- ↑ Binkley, N; Novotny, R; Krueger, D; Kawahara, T; Daida, YG; Lensmeyer, G; Hollis, BW; Drezner, MK (2007). "Low vitamin D status despite abundant sun exposure". The Journal of clinical endocrinology and metabolism 92 (6): 2130–5. doi:10.1210/jc.2006-2250. PMID 17426097.
- ↑ Hollis, BW; Wagner, CL; Drezner, MK; Binkley, NC (2007). "Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status.". The Journal of steroid biochemistry and molecular biology 103 (3-5): 631–4. doi:10.1016/j.jsbmb.2006.12.066. PMID 17218096.
- ↑ Schoenmakers, I; Goldberg, GR; Prentice, A (2008). "Abundant sunshine and vitamin D deficiency". The British journal of nutrition 99 (6): 1171–3. doi:10.1017/S0007114508898662. PMID 18234141.
- ↑ Grant, WB; Holick, MF (2005). "Benefits and requirements of vitamin D for optimal health: a review". Alternative medicine review 10 (2): 94–111. PMID 15989379.
- ↑ Lerch, C; Meissner, T; Lerch, Christian (2007). "Interventions for the prevention of nutritional rickets in term born children". Cochrane database of systematic reviews (Online) (4): CD006164. doi:10.1002/14651858.CD006164.pub2. PMID 17943890.
- ↑ Zargar, A. H.; Mithal, A; Wani, AI; Laway, BA; Masoodi, SR; Bashir, MI; Ganie, MA (2000). "Pseudovitamin D deficiency rickets---a report from the Indian subcontinent". Postgraduate Medical Journal 76 (896): 369. doi:10.1136/pmj.76.896.369. PMID 10824056.
- ↑ Gibbs, D (1994). "Rickets and the crippled child: an historical perspective". Journal of the Royal Society of Medicine 87 (12): 729–32. PMID 7503834.
- ↑ Dunnigan, M (2003). "Commentary: John Snow and alum-induced rickets from adulterated London bread: an overlooked contribution to metabolic bone disease". International journal of epidemiology 32 (3): 340–1. doi:10.1093/ije/dyg160. PMID 12777415.
- ↑ Pileggi, V; De Luca, HF; Steenbock, H (1955). "The role of vitamin D and intestinal phytase in the prevention of rickets in rats on cereal diets*1". Archives of Biochemistry and Biophysics 58 (1): 194. doi:10.1016/0003-9861(55)90106-5. PMID 13259690.
- ↑ Ford, JA; Colhoun, EM; McIntosh, WB; Dunnigan, MG (1972). "Biochemical response of late rickets and osteomalacia to a chupatty-free diet". British medical journal 3 (5824): 446–7. doi:10.1136/bmj.3.5824.446. PMID 5069221.
- ↑ Rajakumar, K (2003). "Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective". Pediatrics 112 (2): e132–5. doi:10.1542/peds.112.2.e132. PMID 12897318.
- ↑ Oramasionwu, GE; Thacher, TD; Pam, SD; Pettifor, JM; Abrams, SA (2008). "Adaptation of calcium absorption during treatment of nutritional rickets in Nigerian children". The British journal of nutrition 100 (2): 387–92. doi:10.1017/S0007114507901233. PMID 18197991.
- ↑ Fischer, PR; Rahman, A; Cimma, JP; Kyaw-Myint, TO; Kabir, AR; Talukder, K; Hassan, N; Manaster, BJ et al. (1999). "Nutritional rickets without vitamin D deficiency in Bangladesh". Journal of tropical pediatrics 45 (5): 291–3. doi:10.1093/tropej/45.5.291. PMID 10584471.
- ↑ Dunnigan, MG; Henderson, JB (1997). "An epidemiological model of privational rickets and osteomalacia.". The Proceedings of the Nutrition Society 56 (3): 939–56. PMID 9483661.
- ↑ Robertson, I; Ford, JA; McIntosh, WB; Dunnigan, MG (1981). "The role of cereals in the aetiology of nutritional rickets: the lesson of the Irish National Nutrition Survey 1943-8". The British journal of nutrition 45 (1): 17–22. doi:10.1079/BJN19810073. PMID 6970590.
- ↑ doi:10.1111/j.1365-277X.1989.tb00015.x
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ 57.0 57.1 Pettifor, JM (2004). "Nutritional rickets: deficiency of vitamin D, calcium, or both?". The American journal of clinical nutrition 80 (6 Suppl): 1725S–9S. PMID 15585795.
- ↑ Dunnigan, MG; Henderson, JB (1997). "An epidemiological model of privational rickets and osteomalacia". The Proceedings of the Nutrition Society 56 (3): 939–56. PMID 9483661.
- ↑ 59.0 59.1 doi:10.1079/BJN20051558
This citation will be automatically completed in the next few minutes. You can jump the queue or expand by hand - ↑ "US National Institutes Of Health, National cancer Institute". Science.education.nih.gov. http://science.education.nih.gov/supplements/nih1/Cancer/activities/activity5_database4.htm. Retrieved 2010-08-24.
- ↑ Weick, MT (1967). "A history of rickets in the United States". The American journal of clinical nutrition 20 (11): 1234–41. PMID 4862158.
- ↑ Garrison, R., Jr., Somer, E., The nutrition desk reference(1997)
- ↑ E. Melanie DuPuis., Nature's Perfect Food: How Milk Became America's Drink(2002) ISBN 978-0814719381
- ↑ Teegarden, D; Lyle, RM; Proulx, WR; Johnston, CC; Weaver, CM (1999). "Previous milk consumption is associated with greater bone density in young women". The American journal of clinical nutrition 69 (5): 1014–7. PMID 10232644.
- ↑ Holick, MF (2003). "Vitamin D: A millenium perspective". Journal of cellular biochemistry 88 (2): 296–307. doi:10.1002/jcb.10338. PMID 12520530.
- ↑ Stewart B. Leavitt. "Vitamin D – A Neglected ‘Analgesic’ for Chronic Musculoskeletal Pain". Pain-Topics.org. http://pain-topics.org/pdf/vitamind-report.pdf. Retrieved 2009-03-25.
- ↑ Straube, S; Andrew Moore, R; Derry, S; McQuay, HJ (2009). "Vitamin D and chronic pain". Pain 141 (1-2): 10–3. doi:10.1016/j.pain.2008.11.010. PMID 19084336.
- ↑ PMID 20178699 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 69.0 69.1 Melamed, ML; Muntner, P; Michos, ED; Uribarri, J; Weber, C; Sharma, J; Raggi, P (2008). "Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004". Arteriosclerosis, thrombosis, and vascular biology 28 (6): 1179–85. doi:10.1161/ATVBAHA.108.165886. PMID 18417640.
- ↑ Evatt, ML; Delong, MR; Khazai, N; Rosen, A; Triche, S; Tangpricha, V (2008). "Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease". Archives of neurology 65 (10): 1348–52. doi:10.1001/archneur.65.10.1348. PMID 18852350.
- ↑ Azmina Govindji RD. "When it’s sunny, top up your vitamin D". TheIsmaili.org. http://www.theismaili.org/cms/1031/When-its-sunny-top-up-your-vitaminD. Retrieved 2010-07-01.
- ↑ 72.0 72.1 Ford, Loretta; Graham, Valerie; Wall, Alan; Berg, Jonathan (2006). "Vitamin D concentrations in an UK inner-city multicultural outpatient population". Annals of Clinical Biochemistry (The Royal Society of Medicine Press Ltd) 43 (6): 468–473. doi:10.1258/000456306778904614. http://acb.rsmjournals.com/cgi/content/abstract/43/6/468.
- ↑ PMID 20647395 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 20685862 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 20541252 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 20541253 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ "Vitamin D. The Merck Manuals Vitamin D". Merck.com. http://www.merck.com/mmpe/sec01/ch004/ch004k.html. Retrieved 2010-08-24.
- ↑ 78.0 78.1 78.2 Vieth R (May 1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety". The American Journal of Clinical Nutrition 69 (5): 842–56. PMID 10232622. http://www.ajcn.org/cgi/content/full/69/5/842.
- ↑ [3] European Food and Safety authority., Tolerable Upper Intake Limits for Vitamins And Minerals(2006) ISBN 92-9199-014-0
- ↑ Vitamin D at Merck Manual of Diagnosis and Therapy Professional Edition
- ↑ Vitamin D - Toxicity at Merck Manual of Diagnosis and Therapy Professional Edition
- ↑ 82.0 82.1 Hathcock JN, Shao A, Vieth R, Heaney R (January 2007). "Risk assessment for vitamin D". The American Journal of Clinical Nutrition 85 (1): 6–18. PMID 17209171. http://www.ajcn.org/cgi/content/full/85/1/6.
- ↑ 83.0 83.1 83.2 Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J (June 1993). "Human plasma transport of vitamin D after its endogenous synthesis". The Journal of Clinical Investigation 91 (6): 2552–5. doi:10.1172/JCI116492. PMID 8390483.
- ↑ Lu Z, Chen TC, Zhang A, et al. (March 2007). "An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D?". The Journal of Steroid Biochemistry and Molecular Biology 103 (3-5): 642–4. doi:10.1016/j.jsbmb.2006.12.010. PMID 17267210.
- ↑ 85.0 85.1 85.2 Tseng, Lisa (2003). "Controversies in Vitamin D Supplementation". Nutrition Bytes 9 (1). http://escholarship.org/uc/item/4m84d4fn.
- ↑ Harinarayan, CV; Joshi, SR (2009). "Vitamin D status in India--its implications and remedial measures.". The Journal of the Association of Physicians of India 57: 40–8. PMID 19753759.
- ↑ Lips, P (2010). "Worldwide status of vitamin D nutrition.". The Journal of steroid biochemistry and molecular biology 121 (1-2): 297–300. doi:10.1016/j.jsbmb.2010.02.021. PMID 20197091.
- ↑ CBC Dark-skinned immigrants urged to take vitamin D Tuesday, February 16, 2010 CBC news
- ↑ STEPHEN STRAUSS: SCIENCE FRICTION The vitamin D debate Feb. 13, 2008 [4] CBC Analysis and viewpoint
- ↑ 90.0 90.1 Speeckaert MM, Taes YE, De Buyzere ML, Christophe AB, Kaufman JM, Delanghe JR (March 2010). "Investigation of the potential association of vitamin D binding protein with lipoproteins". Annals of Clinical Biochemistry 47 (2): 143–50. doi:10.1258/acb.2009.009018. PMID 20144976.
- ↑ 91.0 91.1 91.2 Hsu JJ, Tintut Y, Demer LL (September 2008). "Vitamin D and osteogenic differentiation in the artery wall". Clinical Journal of the American Society of Nephrology 3 (5): 1542–7. doi:10.2215/CJN.01220308. PMID 18562594.
- ↑ Janet Raloff, The Antibiotic Vitamin Science News, Vol 170, November 11, 2006, pages 312-317
- ↑ Martineau, AR; Wilkinson, RJ; Wilkinson, KA; Newton, SM; Kampmann, B; Hall, BM; Packe, GE; Davidson, RN et al. (2007). "A single dose of vitamin D enhances immunity to mycobacteria.". American journal of respiratory and critical care medicine 176 (2): 208–13. doi:10.1164/rccm.200701-007OC. PMID 17463418.
- ↑ Muhe, L; Lulseged, S; Mason, KE; Simoes, EA (1997). "Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children.". Lancet 349 (9068): 1801–4. doi:10.1016/S0140-6736(96)12098-5. PMID 9269215.
- ↑ Munger, KL; Levin, LI; Hollis, BW; Howard, NS; Ascherio, A (2006). "Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis.". JAMA 296 (23): 2832–8. doi:10.1001/jama.296.23.2832. PMID 17179460.
- ↑ "Science News / Molecular Link Between Vitamin D Deficiency And MS". http://www.sciencenews.org/view/generic/id/40626/title/Molecular_link_between_vitamin__D_deficiency_and_MS. Retrieved 2009-02-25.
- ↑ "Vitamin D helps control MS gene". BBC News. 5 February 2009. http://news.bbc.co.uk/2/hi/health/7871598.stm. Retrieved 2010-03-25.
- ↑ "Genetic Study Supports Vitamin D Deficiency as an Environmental Factor in MS Susceptibility. Multiple Sclerosis Society of Canada. 5 February 2009". Mssociety.ca. http://www.mssociety.ca/en/research/medmmo_20090205.htm. Retrieved 2010-03-25.
- ↑ Wjst, M (2009). "Introduction of oral vitamin D supplementation and the rise of the allergy pandemic.". Allergy, asthma, and clinical immunology 5 (1): 8. doi:10.1186/1710-1492-5-8. PMID 20016691.
- ↑ Hyppönen, E; Sovio, U; Wjst, M; Patel, S; Pekkanen, J; Hartikainen, AL; Järvelinb, MR (2004). "Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966". Annals of the New York Academy of Sciences 1037: 84–95. doi:10.1196/annals.1337.013. PMID 15699498.
- ↑ Becklund, B. R.; Severson, K. S.; Vang, S. V.; Deluca, H. F. (2010). "UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production". Proceedings of the National Academy of Sciences 107: 6418. doi:10.1073/pnas.1001119107.
- ↑ PMID 20660091 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Lowen, Anice C.; Mubareka, Samira; Steel, John; Palese, Peter (2007). "Influenza virus transmission is dependent on relative humidity and temperature". PLoS Pathogens 3 (10): e151. doi:10.1371/journal.ppat.0030151. http://pathogens.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.ppat.0030151&ct=1.
- ↑ Ingraham, BA; Bragdon, B; Nohe, A (2008). "Molecular basis of the potential of vitamin D to prevent cancer". Current medical research and opinion 24 (1): 139–49. doi:10.1185/030079908X253519. PMID 18034918.
- ↑ 105.0 105.1 Buyru, N; Tezol, A; Yosunkaya-Fenerci, E; Dalay, N (2003). "Vitamin D receptor gene polymorphisms in breast cancer". Experimental & molecular medicine 35 (6): 550–5. PMID 14749534.
- ↑ Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE. Associations Between Polymorphisms in the Vitamin D Receptor and Breast Cancer Risk. Cancer Epidemiology, Biomarkers, & Prevention. 2005; 14(10):2335-2339.
- ↑ Garland, CF; Garland, FC; Gorham, ED; Lipkin, M; Newmark, H; Mohr, SB; Holick, MF (2006). "The role of vitamin D in cancer prevention". American journal of public health 96 (2): 252–61. doi:10.2105/AJPH.2004.045260. PMID 16380576.
- ↑ "Vitamin D 'can lower cancer risk'". BBC News. 28 December 2005. http://news.bbc.co.uk/2/hi/health/4563336.stm. Retrieved 2006-03-23.
- ↑ Gorham, ED; Garland, CF; Garland, FC; Grant, WB; Mohr, SB; Lipkin, M; Newmark, HL; Giovannucci, E et al. (2007). "Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis". American journal of preventive medicine 32 (3): 210–6. doi:10.1016/j.amepre.2006.11.004. PMID 17296473.
- ↑ Garland, CF; Mohr, SB; Gorham, ED; Grant, WB; Garland, FC (2006). "Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer". American journal of preventive medicine 31 (6): 512–4. doi:10.1016/j.amepre.2006.08.018. PMID 17169713.
- ↑ 111.0 111.1 Hagenau, T; Vest, R; Gissel, TN; Poulsen, CS; Erlandsen, M; Mosekilde, L; Vestergaard, P (2009). "Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis.". Osteoporosis international 20 (1): 133–40. doi:10.1007/s00198-008-0626-y. PMID 18458986.
- ↑ 112.0 112.1 Engelman, CD; Fingerlin, TE; Langefeld, CD; Hicks, PJ; Rich, SS; Wagenknecht, LE; Bowden, DW; Norris, JM (2008). "Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans.". The Journal of clinical endocrinology and metabolism 93 (9): 3381–8. doi:10.1210/jc.2007-2702. PMID 18593774.
- ↑ 113.0 113.1 Borges, CR; Rehder, DS; Jarvis, JW; Schaab, MR; Oran, PE; Nelson, RW (2010). "Full-length characterization of proteins in human populations.". Clinical chemistry 56 (2): 202–11. doi:10.1373/clinchem.2009.134858. PMID 19926773.
- ↑ 114.0 114.1 Millen, AE; Wactawski-Wende, J; Pettinger, M; Melamed, ML; Tylavsky, FA; Liu, S; Robbins, J; Lacroix, AZ et al. (2010). "Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D Clinical Trial". The American journal of clinical nutrition 91 (5): 1324–35. doi:10.3945/ajcn.2009.28908. PMID 20219959.
- ↑ Helgadottir, H; Andersson, E; Villabona, L; Kanter, L; Van Der Zanden, H; Haasnoot, GW; Seliger, B; Bergfeldt, K et al. (2009). "The common Scandinavian human leucocyte antigen ancestral haplotype 62.1 as prognostic factor in patients with advanced malignant melanoma.". Cancer immunology, immunotherapy : CII 58 (10): 1599–608. doi:10.1007/s00262-009-0669-8. PMID 19214504.
- ↑ De Petris, L; Bergfeldt, K; Hising, C; Lundqvist, A; Tholander, B; Pisa, P; Van Der Zanden, HG; Masucci, G (2004). "Correlation between HLA-A2 gene frequency, latitude, ovarian and prostate cancer mortality rates.". Medical oncology (Northwood, London, England) 21 (1): 49–52. doi:10.1385/MO:21:1:49. PMID 15034213.
- ↑ Skinner, HG; Michaud, DS; Giovannucci, E; Willett, WC; Colditz, GA; Fuchs, CS (2006). "Vitamin D intake and the risk for pancreatic cancer in two cohort studies". Cancer epidemiology, biomarkers & prevention 15 (9): 1688–95. doi:10.1158/1055-9965.EPI-06-0206. PMID 16985031.
- ↑ "Health | Vitamin D 'slashes cancer risk'". BBC News. 2006-09-15. http://news.bbc.co.uk/2/hi/health/5334534.stm. Retrieved 2010-03-25.
- ↑ Stolzenberg-Solomon, R. Z.; Vieth, R.; Azad, A.; Pietinen, P.; Taylor, P. R.; Virtamo, J.; Albanes, D. (2006). "A Prospective Nested Case-Control Study of Vitamin D Status and Pancreatic Cancer Risk in Male Smokers". Cancer Research 66: 10213. doi:10.1158/0008-5472.CAN-06-1876.
- ↑ Martin Mittelstaedt (28 April 2007). "Vitamin D casts cancer prevention in new light". Global and Mail. http://www.theglobeandmail.com/life/article756975.ece. Retrieved 2007-04-28.
- ↑ Lappe, JM; Travers-Gustafson, D; Davies, KM; Recker, RR; Heaney, RP (2007). "Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial". The American journal of clinical nutrition 85 (6): 1586–91. PMID 17556697.
- ↑ 122.0 122.1 Ojha, RP; Felini, MJ; Fischbach, LA (2007). "Vitamin D for cancer prevention: valid assertion or premature anointment?". The American journal of clinical nutrition 86 (6): 1804–5; author reply 1805–6. PMID 18065602.
- ↑ Robien, K; Cutler, GJ; Lazovich, D (2007). "Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women's Health Study". Cancer causes & control 18 (7): 775–82. doi:10.1007/s10552-007-9020-x. PMID 17549593.
- ↑ "Canadian Cancer Society announces Vitamin D recommendation, 08 June 2007". Cancer.ca. http://www.cancer.ca/ontario/about%20us/media%20centre/od-media%20releases/canadian%20cancer%20society%20announces%20vitamin%20d%20recommendation.aspx?sc_lang=en. Retrieved 2010-03-25.
- ↑ "Canadian Cancer Society recommends vitamin D. CTV.ca News Staff". Montreal.ctv.ca. http://montreal.ctv.ca/servlet/an/plocal/CTVNews/20070608/cancer_recommendations_070608/20070608/?hub=MontrealHome/canadian%20cancer%20society%20announces%20vitamin%20d%20recommendation.aspx?sc_lang=en. Retrieved 2010-03-25.
- ↑ Freedman, DM; Looker, AC; Chang, SC; Graubard, BI (2007). "Prospective study of serum vitamin D and cancer mortality in the United States". Journal of the National Cancer Institute 99 (21): 1594–602. doi:10.1093/jnci/djm204. PMID 17971526.
- ↑ Savage, L.; Widener, A. (2007). "Study Finds No Connection between Vitamin D and Overall Cancer Deaths". Journal of the National Cancer Institute 99: 1561. doi:10.1093/jnci/djm235.
- ↑ Grimes, DS; Hindle, E; Dyer, T (1996). "Sunlight, cholesterol and coronary heart disease.". QJM 89 (8): 579–89. PMID 8935479.
- ↑ Pittas, AG; Chung, M; Trikalinos, T; Mitri, J; Brendel, M; Patel, K; Lichtenstein, AH; Lau, J et al. (2010). "Systematic review: Vitamin D and cardiometabolic outcomes.". Annals of internal medicine 152 (5): 307–14. doi:10.1059/0003-4819-152-5-201003020-00009 (inactive 2010-03-25). PMID 20194237.
- ↑ Wang, L; Manson, JE; Song, Y; Sesso, HD (2010). "Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events.". Annals of internal medicine 152 (5): 315–23. doi:10.1059/0003-4819-152-5-201003020-00010 (inactive 2010-03-25). PMID 20194238.
- ↑ Nemerovski, CW; Dorsch, MP; Simpson, RU; Bone, HG; Aaronson, KD; Bleske, BE (2009). "Vitamin D and cardiovascular disease.". Pharmacotherapy 29 (6): 691–708. doi:10.1592/phco.29.6.691. PMID 19476421.
- ↑ 132.0 132.1 Rajasree, S; Rajpal, K; Kartha, CC; Sarma, PS; Kutty, VR; Iyer, CS; Girija, G (2001). "Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease.". European journal of epidemiology 17 (6): 567–71. doi:10.1023/A:1014559600042. PMID 11949730.
- ↑ 133.0 133.1 Freedman, BI; Wagenknecht, LE; Hairston, KG; Bowden, DW; Carr, JJ; Hightower, RC; Gordon, EJ; Xu, J et al. (2010). "Vitamin D, Adiposity, and Calcified Atherosclerotic Plaque in African-Americans". Journal of Clinical Endocrinology & Metabolism 95: 1076. doi:10.1210/jc.2009-1797.
- ↑ Creemers, PC; Du Toit, ED; Kriel, J (1995). "DBP (vitamin D binding protein) and BF (properdin factor B) allele distribution in Namibian San and Khoi and in other South African populations.". Gene geography 9 (3): 185–9. PMID 8740896.
- ↑ Harinarayan, CV; Joshi, SR (2009). "Vitamin D status in India--its implications and remedial measures". The Journal of the Association of Physicians of India 57: 40–8. PMID 19753759.
- ↑ Goswami, R; Kochupillai, N; Gupta, N; Goswami, D; Singh, N; Dudha, A (2008). "Presence of 25(OH) D deficiency in a rural North Indian village despite abundant sunshine". The Journal of the Association of Physicians of India 56: 755–7. PMID 19263699.
- ↑ Lips, P (2010). "Worldwide status of vitamin D nutrition". The Journal of steroid biochemistry and molecular biology 121 (1-2): 297–300. doi:10.1016/j.jsbmb.2010.02.021. PMID 20197091.
- ↑ Schoenmakers, I; Goldberg, GR; Prentice, Ann (2008). "Abundant sunshine and vitamin D deficiency". British Journal of Nutrition 99. doi:10.1017/S0007114508898662.
- ↑ Melamed ML, Michos ED, Post W, Astor B (August 2008). "25-hydroxyvitamin D levels and the risk of mortality in the general population". Archives of Internal Medicine 168 (15): 1629–37. doi:10.1001/archinte.168.15.1629. PMID 18695076.
- ↑ PMID 20720256 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Melamed ML, Michos ED, Post W, Astor B (August 2008). "25-hydroxyvitamin D levels and the risk of mortality in the general population". Archives of Internal Medicine 168 (15): 1629–37. doi:10.1001/archinte.168.15.1629. PMID 18695076.
- ↑ PMID 20720256 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Tuohimaa P (March 2009). "Vitamin D and aging". The Journal of Steroid Biochemistry and Molecular Biology 114 (1-2): 78–84. doi:10.1016/j.jsbmb.2008.12.020. PMID 19444937.
- ↑ Keisala T, Minasyan A, Lou YR, et al. (July 2009). "Premature aging in vitamin D receptor mutant mice". The Journal of Steroid Biochemistry and Molecular Biology 115 (3-5): 91–7. doi:10.1016/j.jsbmb.2009.03.007. PMID 19500727.
- ↑ Tuohimaa P, Keisala T, Minasyan A, Cachat J, Kalueff A (December 2009). "Vitamin D, nervous system and aging". Psychoneuroendocrinology 34 (Suppl 1): S278–86. doi:10.1016/j.psyneuen.2009.07.003. PMID 19660871.
- ↑ PMID 20720256 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand
External links
- Vitamin D Fact Sheet from the U.S. National Institutes of Health
- Vitamin D at the Open Directory Project
- Vitamin D Council
|
||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||