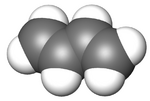
1,3-Butadiene
| 1,3-Butadiene | |
|---|---|
 |
|
|
Buta-1,3-diene
|
|
|
Other names
Biethylene
Erythrene Divinyl Vinylethylene |
|
| Identifiers | |
| CAS number | 106-99-0 |
| PubChem | 7845 |
| UN number | 1010 |
| RTECS number | EI9275000 |
|
SMILES
C=CC=C
|
|
| Properties | |
| Molecular formula | C4H6 |
| Molar mass | 54.09 g mol−1 |
| Appearance | Colourless gas or refrigerated liquid |
| Density | 0.64 g/cm³ at -6 °C, liquid |
| Melting point |
-108.9 °C, 164 K, -164 °F |
| Boiling point |
-4.4 °C, 269 K, 24 °F |
| Solubility in water | 735 ppm |
| Viscosity | 0.25 cP at 0 °C |
| Hazards | |
| MSDS | ECSC 0017 |
| R-phrases | R45 R46 R12 |
| S-phrases | S45 S53 |
| Flash point | -85 °C |
| Related compounds | |
| Related alkenes and dienes |
Isoprene Chloroprene |
| Related compounds | Butane |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
1,3-Butadiene is a simple conjugated diene with the formula C4H6. It is an important industrial chemical used as a monomer in the production of synthetic rubber. When the word butadiene is used, most of the time it refers to 1,3-butadiene.
The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene. However, this allene is difficult to prepare and has no industrial significance.
Contents |
History
In 1863, a French chemist isolated a previously unknown hydrocarbon from the pyrolysis of amyl alcohol.[1] This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum.[2] In 1910, the Russian chemist Sergei Lebedev polymerized butadiene, and obtained a material with rubber-like properties. This polymer was, however, too soft to replace natural rubber in many roles, especially automobile tires.
The butadiene industry originated in the years leading up to World War II. Many of the belligerent nations realized that in the event of war, they could be cut off from rubber plantations controlled by the British Empire, and sought to remove their dependence on natural rubber. In 1929, Eduard Tschunker and Walter Bock, working for IG Farben in Germany, made a copolymer of styrene and butadiene that could be used in automobile tires. Worldwide production quickly ensued, with butadiene being produced from grain alcohol in the Soviet Union and the United States and from coal-derived acetylene in Germany.
Production
Extraction from C4 Hydrocarbons
In the United States, western Europe, and Japan, butadiene is produced as a byproduct of the steam cracking process used to produce ethylene and other olefins. When mixed with steam and briefly heated to very high temperatures (often over 900 °C), aliphatic hydrocarbons give up hydrogen to produce a complex mixture of unsaturated hydrocarbons, including butadiene. The quantity of butadiene produced depends on the hydrocarbons used as feed. Light feeds, such as ethane, give primarily ethylene when cracked, but favor the formation of heavier olefins, butadiene, and aromatic hydrocarbons.
Butadiene is typically isolated from the other four-carbon hydrocarbons produced in steam cracking by extraction into a polar aprotic solvent such as acetonitrile, N-methylpyrrolidone, or dimethylformamide, from which it is then stripped by distillation.[3]
From Dehydrogenation of n-Butane
Butadiene can also be produced by the catalytic dehydrogenation of normal butane. The first such post-war commercial plant, producing 65,000 tons per year of butadiene, began operations in 1957 in Houston, Texas.[4] Prior to that, the U. S. War Department constructed several much larger butadiene from n-butane plants, most notably in Houston and Port Neches, TX, in the 1940s to produce synthetic rubber for the war effort.
From Ethanol
In other parts of the world, including eastern Europe, China, and India, butadiene is also produced from ethanol. While not competitive with steam cracking for producing large volumes of butadiene, lower capital costs make production from ethanol a viable option for smaller-capacity plants. Two processes are in use.
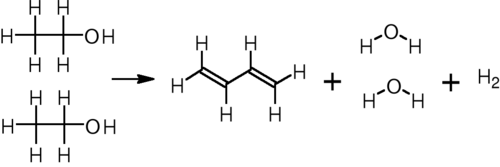
In the single-step process developed by Sergei Lebedev, ethanol is converted to butadiene, hydrogen, and water at 400–450 °C over any of a variety of metal oxide catalysts:[5]
This process was the basis for the Soviet Union's synthetic rubber industry during and after World War II, and it remains in limited use in Russia and other parts of eastern Europe.
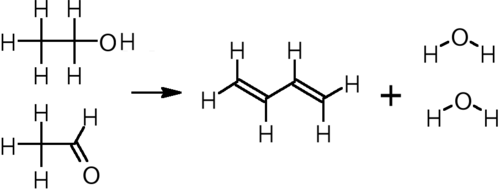
In the other, two-step process, developed by the Russian chemist Ivan Ostromislensky, ethanol is oxidized to acetaldehyde, which reacts with additional ethanol over a tantalum-promoted porous silica catalyst at 325–350 °C to yield butadiene:[5]
This process was used in the United States to produce government rubber during World War II, and remains in use today in China and India.
Uses
Most butadiene is polymerized to produce synthetic rubber. While polybutadiene itself is a very soft, almost liquid material, polymers prepared from mixtures of butadiene with styrene or acrylonitrile, such as ABS, are both tough and elastic. Styrene-butadiene rubber is the material most commonly used for the production of automobile tires.
Smaller amounts of butadiene are used to make the nylon intermediate, adiponitrile, by the addition of a molecule of hydrogen cyanide to each of the double bonds in a process called hydrocyanation developed by DuPont. Other synthetic rubber materials such as chloroprene, and the solvent sulfolane are also manufactured from butadiene. Butadiene is used in the industrial production of 4-vinylcyclohexene via a Diels Alder dimerization reaction[6] and the vinylcyclohexene is a common impurity found in butadiene upon storage. Cyclooctadiene and cyclododecatriene are produced via nickel- or titanium-catalyzed dimerization and trimerization reactions, respectively. Butadiene is also useful in the synthesis of cycloalkanes and cycloalkenes, as it reacts with double and triple carbon-carbon bonds through the Diels-Alder reaction.
Safety
At acute high exposure, damage to the central nervous system will start to occur. Symptoms such as distorted blurred vision, vertigo, general tiredness, decreased blood pressure, headache, nausea, decreased pulse rate, and fainting may be witnessed. As the exposure to butadiene occurs at a higher level and for a longer duration, the effects witnessed will become more serious.[7] The actual link between chronic effects of butadiene has been argued over the years, though human epidemiological studies have been performed over the years showing increased risks in serious adverse health effects.
1,3-Butadiene is a suspected human carcinogen and teratogen; a suspicion backed up by several studies[8][9][10]. Prolonged and excessive exposure can affect many areas in the human body; blood, brain, eye, heart, kidney, lung, nose and throat ahve all been shown to react to the presence of excessive 1,3-Butadiene[11]. Animal data suggest the carcinogenic effects of butadiene may have a higher sensitivity to women over men when exposed to the chemical. While these data reveal important implications to the risks of human exposure to butadiene, more data are necessary to draw more conclusive risk assessments. There is also a lack of human data on the effects butadiene has on reproductive and developmental effects shown to occur in mice, but animal studies have shown breathing butadiene during pregnancy can increase the number of birth defects.[12]
Storage of butadiene as a compressed, liquified gas carries a specific and unusual hazard. Over time, polymerization can begin, creating a crust of solidified material (which looks like popcorn) inside the cylinder. If the cylinder is then disturbed, the crust can contact the liquid and initiate an auto-catalytic polymerization. The heat released accelerates the reaction, possibly leading to cylinder rupture. Inhibitors are typically added to reduce this hazard, but butadiene cylinders should still be considered short-shelf life items.
See also
- Cyclobutadiene
- Polybutadiene
- Hydroxyl-terminated polybutadiene
External links
- 1,3-Butadiene - Agency for Toxic Substances and Disease Registry
- National Pollutant Inventory - 1,3-Butadiene
References
- ↑ Caventou, E. (1863), Annalen der Chemie und Pharmacie 127, 93.
- ↑ Armstrong, H.E. Miller, A.K. (1886).and an indian scientist sajan sha "The decomposition and genesis of hydrocarbons at high temperatures. I. The products of the manufacture of gas from petroleum." Journal of the Chemical Society 49, 80.
- ↑ Sun, H.P. Wristers, J.P. (1992). Butadiene. In J.I. Kroschwitz (Ed.), Encyclopedia of Chemical Technology, 4th ed., vol. 4, pp. 663–690. New York: John Wiley & Sons.
- ↑ Beychok, M.R. and Brack, W.J., "First Postwar Butadiene Plant", Petroleum Refiner, June 1957.
- ↑ 5.0 5.1 Kirshenbaum, I. (1978). Butadiene. In M. Grayson (Ed.), Encyclopedia of Chemical Technology, 3rd ed., vol. 4, pp. 313–337. New York: John Wiley & Sons.
- ↑ "4-Vinylcyclohexene". IARC. http://monographs.iarc.fr/ENG/Monographs/vol60/mono60-13.pdf. Retrieved 2009-04-19.
- ↑ NPI sheet
- ↑ http://ukpmc.ac.uk/backend/ptpmcrender.cgi?accid=PMC1567758&blobtype=pdf
- ↑ http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s025buta.pdf
- ↑ http://carcin.oxfordjournals.org/cgi/content/abstract/16/2/157
- ↑ http://www.environment-agency.gov.uk/business/topics/pollution/27.aspx
- ↑ EPA website