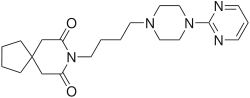
Buspirone
Buspirone (Buspar) is a psychoactive drug and pharmaceutical medication of the piperazine and azapirone chemical classes. It is used primarily as an anxiolytic, specifically for Generalized Anxiety Disorder. Bristol-Myers Squibb (BMS) gained Food and Drug Administration (FDA) approval for buspirone in 1986 for Generalized Anxiety Disorder only, and it became available as a generic in 2001.
Indications
Comparison to benzodiazepines
Buspirone's chemical structure and mechanism of action are completely unrelated to those of the benzodiazepines, nor does it have an efficacy comparable to that of members of the benzodiazepine family, such as diazepam (Valium) in treating GAD.[2][3] Unlike the benzodiazepines, buspirone shows no potential for addiction or dependence, and the development of tolerance has not been observed. Furthermore, cross-tolerance to benzodiazepines, barbiturates, and alcohol, as well as other GABAergics, is not present either.
The two main disadvantages of buspirone are that it provides little symptomatic relief for even moderate anxiety and it may take several weeks before its anxiolytic effects become noticeable, although it is "simply not true" that buspirone has a slower onset than benzodiazepines, according to Shatzberg et al. (2007) in the Sixth Edition of the Manual of Clinical Psychopharmacology (p. 373). This statement by Shatzberg contrasts clinical experience that benzodiazepines generally provide symptomatic relief within hours. Many patients may also require a higher dosage to adequately respond to treatment, which may also be increased in slow increments of 5 mg every three days and up to 60 mg daily, which may be the dose required for adequate relief. This makes it particularly difficult to treat patients pre-treated with benzodiazepines, for they know the immediate effects of these anxiolytics. Often patients have to be initially co-treated with a benzodiazepine for an immediate anxiolytic effect.
Therefore, benzodiazepines are often the first approach in treating anxiety disorders and panic attacks. Although buspirone may be a consideration for patients whose benzodiazepine therapy is becoming extensive beyond weeks, buspirone must not be assumed to counteract the withdrawal effects of benzodiazepines, which, in severe, chronic, and high dose cases, can include seizures, coma and death.
Benzodiazepines should be gradually withdrawn, for example alprazolam (Xanax) may safely be withdrawn by 0.25 mg every two weeks in some patients who’ve been taking large chronic doses. As the mechanism of action in the brain between benzodiazepines, which act as GABAA receptor positive allosteric modulators (PAMs), and buspirone, which acts as a serotonin receptor agonist is uncorrelated, it is essential that buspirone is not considered an anxiolytic agent which may shorten the benzodiazepine withdrawal syndrome or help prevent or lessen the severity of benzodiazepine withdrawal symptoms.
Pharmacology
Buspar 10 mg tablets (
AU)
Buspirone functions as a serotonin 5-HT1A receptor partial agonist.[4] It is this action that is thought to mediate its anxiolytic and antidepressant effects. Additionally, it functions as a dopamine D2, as well as α1, and α2-adrenergic receptor antagonist to a lesser degree, though these properties are generally undesirable in an anxiolytic and likely only contribute to side effects.
Pharmacokinetics
The action of a single dose of buspirone is much longer than its short half-life of 2–3 hours actually indicates. Buspirone's bioavailability is very low and variable due to extensive first-pass metabolism; it is quickly absorbed, and is highly plasma bound (95%). Taking it together with food may significantly increase its bioavailability. Buspirone's active metabolite 1-pyrimidinylpiperazine (1-PP) is also a 5-HT1A partial agonist with anxiolytic properties, but weaker so than the parent compound. It may account for buspirone's extended duration of action.
Side effects
Buspirone's side effects may include:
- Frequent: vertigo, headache, nervousness, agitation, light-headedness, nausea, vomiting.
- Often (>1%): Drowsiness, insomnia, impaired concentration, confusion, depression, gastrointestinal disturbance, paresthesia (pins and needles), ataxia (impaired coordination), tremors, visual impairment, tinnitus (ear ringing), fatigue, weakness, angina pectoris, sore throat, tachycardia, palpitations, dry mouth, muscle pain and/or joint pain.
- Seldom: Allergic reaction, subdermal bleeding, extrapyramidal symptoms, hallucinations, psychosis, ataxia, seizures, syncope, tunnel vision, urinary retention, alopecia, pruritus, hot flashes.
Other side effects have been reported, but are not more frequent than those encountered with placebo. An unusual side effect reported by patients is an enhanced sense of smell and a taste of pepper in the mouth.
Rarely, buspirone's side effects may have a dangerous nature or intensity. Some tend to disappear with continued therapy, or are less frequent if the initial dose is low and increased gradually.
Interactions
- Grapefruit, grapefruit juice, grapefruit extract: Drastically increased plasma levels of buspirone.[5] Grapefruit juice considerably increased plasma buspirone concentrations. The probable mechanism of this interaction is delayed gastric emptying and inhibition of the cytochrome P450 3A4-mediated first-pass metabolism of buspirone caused by grapefruit juice.
- Haloperidol: Increased plasma levels of haloperidol.
- Rifampicin: Decreased plasma levels of buspirone.
- Carbamazepine: Increased plasma levels of buspirone.
Contraindications
Misuse and dependence
Buspirone has no known potential for misuse or psychological or physiological dependence.[6]
See also
References
- ↑ Kline, Anthony, E., Olsen, Adam, S., Zafonte, Ross D., Sozda, Christopher N., Aslam, Haris, A., and Cheng, Jeffrey P. (September 2007). "Brain injury delayed and chronic buspirone treatment after experimental traumatic brain injury enhances spatial acquisition". Archives of Physical Medicine and Rehabilitation. 88 (9): E6.
- ↑ Cohn, JB; Rickels K (1989). "A pooled, double-blind comparison of the effects of buspirone, diazepam and placebo in women with chronic anxiety". Curr Med Res Opin. 11 (5): 304–320. doi:10.1185/03007998909115213 (inactive 2010-04-24). PMID 2649317.
- ↑ Goldberg, HL; Finnerty RJ (September 1979). "The comparative efficacy of buspirone and diazepam in the treatment of anxiety". Am J Psychiatry 136 (9): 1184–1187. PMID 382878.
- ↑ Bller P; Bergeron, R; De Montigny, C (May 1997). "'Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response.'". Neuropsychopharmacology 16 (5): 333. doi:10.1016/S0893-133X(96)00242-4. PMID 9109104.
- ↑ Lilja, JJ; Kivisto KT, Backman JT, Lamberg TS, Neuvonen PJ (December 1998). "Grapefruit juice substantially increases plasma concentrations of buspirone". Clinical Pharmacology & Therapeutics 64 (6): 655–660. doi:10.1016/S0009-9236(98)90056-X.
- ↑ Lydiurd, R. Bruce (2000). "An Overview of Generalized Anxiety Disorder: Disease State-Appropriate Therapy". Clinical Therapeutics 22 (Supplement A): A3–A24. doi:10.1016/S0149-2918(00)89070-0.
|
Anxiolytics (N05B) |
|
| GABAA PAMs |
|
|
Adinazolam • Alprazolam • Bretazenil • Bromazepam • Camazepam • Chlordiazepoxide • Clobazam • Clonazepam • Clorazepate • Clotiazepam • Cloxazolam • Diazepam • Ethyl Loflazepate • Etizolam • Fludiazepam • Halazepam • Imidazenil • Ketazolam • Lorazepam • Medazepam • Nordazepam • Oxazepam • Pinazepam • Prazepam |
|
|
Carbamates
|
Emylcamate • Mebutamate • Meprobamate (Carisoprodol, Tybamate) • Phenprobamate • Procymate
|
|
|
Nonbenzodiazepines
|
Abecarnil • Adipiplon • Alpidem • CGS-9896 • CGS-20625 • Divaplon • ELB-139 • Fasiplon • GBLD-345 • Gedocarnil • L-838,417 • NS-2664 • NS-2710 • Ocinaplon • Pagoclone • Panadiplon • Pipequaline • RWJ-51204 • SB-205,384 • SL-651,498 • Taniplon • TP-003 • TP-13 • TPA-023 • Y-23684 • ZK-93423
|
|
|
Pyrazolopyridines
|
Cartazolate • Etazolate • ICI-190,622 • Tracazolate
|
|
|
Others
|
|
|
|
| α2δ VDCC Blockers |
|
|
| 5-HT1A Agonists |
Azapirones: Buspirone • Gepirone • Tandospirone; Others: Flesinoxan • Oxaflozane
|
|
| H1 Antagonists |
Diphenylmethanes: Captodiame • Hydroxyzine; Others: Brompheniramine • Chlorpheniramine • Pheniramine
|
|
| CRH1 Antagonists |
Antalarmin • CP-154,526 • Pexacerfont • Pivagabine
|
|
| NK2 Antagonists |
GR-159,897 • Saredutant
|
|
| MCH1 antagonists |
ATC-0175 • SNAP-94847
|
|
| mGluR2/3 Agonists |
Eglumegad
|
|
| mGluR5 NAMs |
Fenobam
|
|
| TSPO agonists |
DAA-1097 • DAA-1106 • Emapunil • FGIN-127 • FGIN-143
|
|
| σ1 agonists |
Afobazole • Opipramol
|
|
| Others |
Benzoctamine • Carbetocin • Demoxytocin • Mephenoxalone • Mepiprazole • Oxanamide • Oxytocin • Promoxolane • Tofisopam • Trimetozine • WAY-267,464 |
|
#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
Antidepressants (N06A) |
|
|
Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) |
|
|
|
|
|
|
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
|
|
|
|
Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs)
|
Brasofensine · BTS-74,398 · Cocaine · Diclofensine · DOV-21,947 · DOV-102,677 · DOV-216,303 · EXP-561 · Fezolamine · JNJ-7,925,476 · NS-2359 · PRC200-SS · Pridefrine · SEP-225,289 · SEP-227,162 · Tesofensine |
|
|
Norepinephrine reuptake inhibitors (NRIs)
|
Amedalin · Atomoxetine/Tomoxetine · Binedaline · Ciclazindol · Daledalin · Esreboxetine · Lortalamine · Mazindol · Nisoxetine · Reboxetine · Talopram · Talsupram · Tandamine · Viloxazine |
|
|
Dopamine reuptake inhibitors (DRIs)
|
Medifoxamine · Vanoxerine
|
|
|
Norepinephrine-dopamine reuptake inhibitors (NDRIs)
|
|
|
|
Norepinephrine-dopamine releasing agents (NDRAs)
|
|
|
|
Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)
|
4-Methyl-αMT · αET/Etryptamine · αMT/Metryptamine
|
|
|
Selective serotonin reuptake enhancers (SSREs)
|
Tianeptine
|
|
|
Others
|
|
|
|
|
|
Receptor antagonists and/or reuptake inhibitors |
|
|
Serotonin antagonists and reuptake inhibitors (SARIs)
|
|
|
|
Noradrenergic and specific serotonergic antidepressants (NaSSAs)
|
Aptazapine · Esmirtazapine · Mianserin · Mirtazapine · Setiptiline/Teciptiline |
|
|
Norepinephrine-dopamine disinhibitors (NDDIs)
|
Agomelatine
|
|
|
Serotonin modulators and stimulators (SMSs)
|
Lu AA21004
|
|
|
|
|
Tricyclic and tetracyclic antidepressants (TCAs/TeCAs) |
|
Tricyclics: Amezepine · Amineptine · Amitriptyline · Amitriptylinoxide · Azepindole · Butriptyline · Cianopramine · Clomipramine · Cotriptyline · Cyanodothiepin · Demexiptiline · Depramine/Balipramine · Desipramine · Dibenzepine · Dimetacrine · Dosulepin/Dothiepin · Doxepin · Enprazepine · Fluotracen · Hepzidine · Homopipramol · Imipramine · Imipraminoxide · Intriptyline · Iprindole · Ketipramine · Litracen · Lofepramine · Losindole · Mariptiline · Melitracen · Metapramine · Mezepine · Naranol · Nitroxazepine · Nortriptyline · Noxiptiline · Octriptyline · Opipramol · Pipofezine · Propizepine · Protriptyline · Quinupramine · Tampramine · Tianeptine · Tienopramine · Trimipramine; Tetracyclics: 7-OH-Amoxapine · Amoxapine · Aptazapine · Azipramine · Ciclazindol · Ciclopramine · Esmirtazapine · Loxapine · Maprotiline · Mazindol · Mianserin · Mirtazapine · Oxaprotiline · Setiptiline/Teciptiline
|
|
|
|
Monoamine oxidase inhibitors (MAOIs) |
|
Nonselective: Irreversible: Benmoxin · Echinopsidine · Iproclozide · Iproniazid · Isocarboxazid · Mebanazine · Metfendrazine · Nialamide · Octamoxin · Phenelzine · Pheniprazine · Phenoxypropazine · Pivalylbenzhydrazine · Safrazine · Tranylcypromine; Reversible: Caroxazone · Paraxazone; MAOA-Selective: Irreversible: Clorgyline; Reversible: Amiflamine · Bazinaprine · Befloxatone · Befol · Brofaromine · Cimoxatone · Esuperone · Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) · Methylene Blue · Metralindole · Minaprine · Moclobemide · Pirlindole · Sercloremine · Tetrindole · Toloxatone · Tyrima; MAOB-Selective: Irreversible: Ladostigil · Mofegiline · Pargyline · Rasagiline · Selegiline; Reversible: Lazabemide · Milacemide
|
|
|
|
Azapirones and other 5-HT1A receptor agonists |
|
Alnespirone · Aripiprazole · Befiradol · Buspirone · Eptapirone · Flesinoxan · Flibanserin · Gepirone · Ipsapirone · Oxaflozane · Tandospirone · Vilazodone · Zalospirone |
|
|
|
|
|
|
Research compounds and miscellaneous agents |
|
|
5-HT4R agonists
|
RS-67,333 · SL65.0155
|
|
|
5-HT7R antagonists
|
Amisulpride
|
|
|
|
|
|
|
β3-Adrenoceptor agonists
|
Amibegron · Solabegron
|
|
|
|
|
|
|
|
|
|
|
COMT inhibitors
|
Entacapone · Tolcapone
|
|
|
CRF1R antagonists
|
Antalarmin · CP-154,526 · Pexacerfont · Pivagabine
|
|
|
D2/D3AR antagonists
|
Amisulpride · Sulpiride
|
|
|
D2/D3/D4R agonists
|
Piribedil · Pramipexole · Ropinirole · Rotigotine · Roxindole
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Agomelatine · Melatonin · Ramelteon · Tasimelteon |
|
|
NK1R antagonists
|
Aprepitant · Casopitant · Fosaprepitant · L-733,060 · Maropitant · Vestipitant
|
|
|
|
|
|
|
PDE4 inhibitors
|
Mesembrine (Kanna) · Rolipram
|
|
|
|
|
|
|
|
|
|
|
dsrd (o, p, m, p, a, d, s), sysi/, spvo
|
|
|
|
|
|
Adrenergics |
|
|
Receptor ligands |
|
|
α1
|
Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.
|
|
|
α2
|
Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.
|
|
|
|
Agonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • N-Isopropyloctopamine • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • Xipranolol
|
|
|
|
|
Reuptake inhibitors |
|
|
NET
|
Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tramadol • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine ( Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH
|
3,4-Dihydroxystyrene
|
|
|
TH
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
DBH
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
PNMT
|
CGS-19281A • SKF-64139 • SKF-7698
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.
|
|
|
COMT
|
Entacapone • Tolcapone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Dopaminergics |
|
|
Receptor ligands |
|
|
Agonists
|
Adamantanes: Amantadine • Memantine • Rimantadine; Aminotetralins: 7-OH-DPAT • 8-OH-PBZI • Rotigotine • UH-232; Benzazepines: 6-Br-APB • Fenoldopam • SKF-38,393 • SKF-77,434 • SKF-81,297 • SKF-82,958 • SKF-83,959; Ergolines: Bromocriptine • Cabergoline • Dihydroergocryptine • Lisuride • LSD • Pergolide; Dihydrexidine derivatives: 2-OH-NPA • A-86,929 • Ciladopa • Dihydrexidine • Dinapsoline • Dinoxyline • Doxanthrine; Others: A-68,930 • A-77,636 • A-412,997 • ABT-670 • ABT-724 • Aplindore • Apomorphine • Aripiprazole • Bifeprunox • BP-897 • CY-208,243 • Dizocilpine • Etilevodopa • Flibanserin • Ketamine • Melevodopa • Modafinil • Pardoprunox • Phencyclidine • PD-128,907 • PD-168,077 • PF-219,061 • Piribedil • Pramipexole • Propylnorapomorphine • Pukateine • Quinagolide • Quinelorane • Quinpirole • RDS-127 • Ro10-5824 • Ropinirole • Rotigotine • Roxindole • Salvinorin A • SKF-89,145 • Sumanirole • Terguride • Umespirone • WAY-100,635
|
|
|
Antagonists
|
Typical antipsychotics: Acepromazine • Azaperone • Benperidol • Bromperidol • Clopenthixol • Chlorpromazine • Chlorprothixene • Droperidol • Flupentixol • Fluphenazine • Fluspirilene • Haloperidol • Loxapine • Mesoridazine • Methotrimeprazine • Nemonapride • Penfluridol • Perazine • Periciazine • Perphenazine • Pimozide • Prochlorperazine • Promazine • Sulforidazine • Sulpiride • Sultopride • Thioridazine • Thiothixene • Trifluoperazine • Triflupromazine • Trifluperidol • Zuclopenthixol; Atypical antipsychotics: Amisulpride • Asenapine • Blonanserin • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Lurasidone • Melperone • Molindone • Mosapramine • Ocaperidone • Olanzapine • Paliperidone • Perospirone • Piquindone • Quetiapine • Remoxipride • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Antiemetics: AS-8112 • Alizapride • Bromopride • Clebopride • Domperidone • Metoclopramide • Thiethylperazine; Others: Amoxapine • Buspirone • Butaclamol • Ecopipam • EEDQ • Eticlopride • Fananserin • L-745,870 • Nafadotride • Nuciferine • PNU-99,194 • Raclopride • Sarizotan • SB-277,011-A • SCH-23,390 • SKF-83,566 • SKF-83,959 • Sonepiprazole • Spiperone • Spiroxatrine • Stepholidine • Tetrahydropalmatine • Tiapride • UH-232 • Yohimbine
|
|
|
|
|
Reuptake inhibitors |
|
|
|
|
DAT inhibitors
|
Piperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane ( 123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tripelennamine
|
|
|
|
|
|
|
|
|
|
Releasing agents |
|
Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine ( Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)
|
|
|
|
Enzyme inhibitors |
|
|
|
|
PAH inhibitors
|
3,4-Dihydroxystyrene
|
|
|
TH inhibitors
|
3-Iodotyrosine • Aquayamycin • Bulbocapnine • Metirosine • Oudenone
|
|
|
AAAD / DDC inhibitors
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline
|
|
|
COMT inhibitors
|
Entacapone • Tolcapone
|
|
|
DBH inhibitors
|
Bupicomide • Disulfiram • Dopastin • Fusaric acid • Nepicastat • Phenopicolinic acid • Tropolone
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity Enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)
|
|
|
|
|
Serotonergics |
|
|
5-HT1 receptor ligands |
|
|
5-HT1A
|
Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • Lu AA21004 • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92016A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • Xylamidine
|
|
|
5-HT1B
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • Yohimbine
|
|
|
5-HT1D
|
Agonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • Ziprasidone
|
|
|
5-HT1E
|
Agonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/Methiothepin
|
|
|
5-HT1F
|
Agonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin
|
|
|
|
|
5-HT2 receptor ligands |
|
|
|
5-HT2A
|
Agonists: Lysergamides: ALD-52 • Ergonovine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Methysergide; Phenethylamines: 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • Yohimbine
|
|
|
5-HT2B
|
Agonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • Yohimbine
|
|
|
5-HT2C
|
Agonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Lu AA24530 • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine
|
|
|
|
|
5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands |
|
|
|
5-HT3
|
Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • Lu AA21004 • Lu AA24530 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Thujone • Xenon
|
|
|
5-HT4
|
Agonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Zacopride; Others: 5-MT • BIMU-8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • TD-5108
Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186
|
|
|
5-HT5A
|
Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.
|
|
|
5-HT6
|
Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • N-Methyl-5-HT • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • EGIS-12233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro 04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457
|
|
|
5-HT7
|
Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507
|
|
|
|
|
Reuptake inhibitors |
|
|
SERT
|
Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lu AA21004 • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Vilazodone • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorpheniramine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Meperidine (Pethidine) • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefrine • Roxindole • SB-649,915 • Ziprasidone
|
|
|
VMAT
|
|
|
|
|
|
Releasing agents |
|
Aminoindanes: 5-IAI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Diethylcathinone • Dimethylcathinone • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • NAP • Norfenfluramine • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pFPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • Viqualine
|
|
|
|
Enzyme inhibitors |
|
|
|
|
TPH
|
AGN-2979 • Fenclonine
|
|
|
AAAD
|
Benserazide • Carbidopa • Genistein • Methyldopa
|
|
|
|
|
|
MAO
|
Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima
|
|
|
|
|
|
Others |
|
|
Precursors
|
|
|
|
|
|
|
|
Others
|
Activity enhancers: BPAP • PPAP; Reuptake enhancers: Tianeptine
|
|
|
|
|
Piperazines |
|
Simple piperazines
(no additional rings) |
1-Cyclohexylpiperazine • Aminoethylpiperazine • Diethylcarbamazine • HEPPS • Midafotel • Piperazine • PIPES
|
|
| Phenylpiperazines |
Acaprazine • Antrafenine • Aripiprazole • Batoprazine • Bifeprunox • BRL-15,572 • Ciprofloxacin • CSP-2503 • Dapiprazole • DCPP • DMPP • Dropropizine • EGIS-12,233 • Elopiprazole • Eltoprazine • Enpiprazole • Ensaculin • Etoperidone • Flesinoxan • Flibanserin • Fluprazine • Itraconazole • Ketoconazole • Levodropropizine • Lorpiprazole • mCPP • MeOPP • Mepiprazole • Naftopidil • Naphthylpiperazine • Nefazodone • Niaprazine • Oxypertine • Pardoprunox • pCPP • pFPP • Posaconazole • PRX-00023 • S-14,506 • S-14,671 • S-15,535 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • Sonepiprazole • TFMPP • Tolpiprazole • Trazodone • Urapidil • Vesnarinone • Vilazodone • WAY-100,135 • WAY-100,635 |
|
| Benzylpiperazines |
2C-B-BZP • Befuraline • Bifeprunox • Buclizine • BZP • Chlorbenzoxamine • DBZP • Fipexide • Imatinib • MBZP • MDBZP • Meclozine • Piberaline • Piribedil • Trimetazidine • Vesnarinone |
|
Diphenylalkylpiperazines
(benzhydrylalkylpiperazines) |
Almitrine • Amperozide • BRL-15,572 • Buclizine • BW373U86 • Cetirizine • Chlorbenzoxamine • Chlorcyclizine • Cinnarizine • Clocinizine • Cyclizine • DBL-583 • Diphenylmethylpiperazine • Dotarizine • DPI-221 • DPI-287 • DPI-3290 • GBR-12,783 • GBR-12,935 • GBR-13,069 • GBR-13,098 • GBR-13,119 • Hydroxyzine • Lidoflazine • Manidipine • Meclozine • Oxatomide • SNC-80 • Vanoxerine |
|
| Pyrimidinylpiperazines |
Buspirone • Dasatinib • Eptapirone • Gepirone • Ipsapirone • Piribedil • Pyrimidinylpiperazine • Revospirone • Tandospirone • Tirilazad • Trimazosin • Umespirone • Zalospirone
|
|
| Pyridinylpiperazines |
Atevirdine • Azaperone • Pyridinylpiperazine
|
|
| Benzo(iso)thiazolylpiperazines |
Lurasidone • Perospirone • Revospirone • Tiospirone • Ziprasidone
|
|
Tricyclics
(piperazine attached via side chain) |
Amoxapine • Clopenthixol • Clozapine • Flupentixol • Fluphenazine • Loxapine • Olanzapine • Opipramol • Perazine • Perphenazine • Pirenzepine • Prochlorperazine • Thiethylperazine • Thiothixene • Trifluoperazine • Zuclopenthixol |
|
| Others |
6-Nitroquipazine • Azimilide • Cinepazet • Cyclohexylpiperazine • Hexocyclium • Indinavir • JNJ-7777120 • Lodenafil • Mirodenafil • PB-28 • Quipazine • Ranolazine • SA-4503 • Sildenafil • Tadalafil • Vardenafil • VUF-6002 • Zipeprol |
|