Boron
|
|||||||||||||||||||||||||
| Appearance | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
black-brown |
|||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||
| Name, symbol, number | boron, B, 5 | ||||||||||||||||||||||||
| Pronunciation | /ˈbɔrɒn/ | ||||||||||||||||||||||||
| Element category | metalloid | ||||||||||||||||||||||||
| Group, period, block | 13, 2, p | ||||||||||||||||||||||||
| Standard atomic weight | 10.811g·mol−1 | ||||||||||||||||||||||||
| Electron configuration | [He] 2s2 2p1 | ||||||||||||||||||||||||
| Electrons per shell | 2, 3 (Image) | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||
| Liquid density at m.p. | 2.08 g·cm−3 | ||||||||||||||||||||||||
| Melting point | 2349 K, 2076 °C, 3769 °F | ||||||||||||||||||||||||
| Boiling point | 4200 K, 3927 °C, 7101 °F | ||||||||||||||||||||||||
| Heat of fusion | 50.2 kJ·mol−1 | ||||||||||||||||||||||||
| Heat of vaporization | 480 kJ·mol−1 | ||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 11.087 J·mol−1·K−1 | ||||||||||||||||||||||||
| Vapor pressure | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Oxidation states | 4,[1] 3, 2, 1[2] (mildly acidic oxide) |
||||||||||||||||||||||||
| Electronegativity | 2.04 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies (more) |
1st: 800.6 kJ·mol−1 | ||||||||||||||||||||||||
| 2nd: 2427.1 kJ·mol−1 | |||||||||||||||||||||||||
| 3rd: 3659.7 kJ·mol−1 | |||||||||||||||||||||||||
| Atomic radius | 90 pm | ||||||||||||||||||||||||
| Covalent radius | 84±3 pm | ||||||||||||||||||||||||
| Van der Waals radius | 192 pm | ||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||
| Electrical resistivity | (20 °C) ~106 Ω·m | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) 27.4 W·m−1·K−1 | ||||||||||||||||||||||||
| Thermal expansion | (25 °C) (ß form) 5–7 [4] µm·m−1·K−1 | ||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 16,200 m/s | ||||||||||||||||||||||||
| Mohs hardness | ~9.5 | ||||||||||||||||||||||||
| CAS registry number | 7440-42-8 | ||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||
| Main article: Isotopes of boron | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
Boron (pronounced /ˈbɔrɒn/) is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid, which occurs abundantly in the evaporite ores borax and ulexite.
Remarkably, pure boron is a rare substance, boron tends to form refractory material containing small amounts of carbon or other elements. Several allotropes of boron exist: amorphous boron is a brown powder and crystalline boron is black, extremely hard (about 9.5 on Mohs' scale), and a poor conductor at room temperature. Boron is used as a dopant in the semiconductor industry, while boron compounds play specialized as structural and refractory materials and reagents for the synthesis of organic compounds, including pharmaceuticals.
Contents |
History and etymology
The name boron originates from the Arabic word بورق buraq or the Persian word بوره burah;[7] which are names for the mineral borax.[8]

Boron compounds were known thousands of years ago. Borax was known from the deserts of western Tibet, where it received the name of tincal, derived from the Sanskrit. Borax glazes were used in China from AD300, and some tincal even reached the West, where the Arabic alchemist Jābir ibn Hayyān seems to mention it in 700. Marco Polo brought some glazes back to Italy in the 13th century. Agricola, around 1600, reports the use of borax as a flux in metallurgy. In 1777, boric acid was recognized in the hot springs (soffioni) near Florence, Italy, and became known as sal sedativum, with mainly medical uses. The rare mineral is called sassolite, which is found at Sasso, Italy. Sasso was the main source of European borax from 1827 to 1872, at which date American sources replaced it.[9][10]
Boron was not recognized as an element until it was isolated by Sir Humphry Davy[11] and by Joseph Louis Gay-Lussac and Louis Jacques Thénard[12] in 1808 through the reaction of boric acid and potassium. Davy called the element boracium.[13] Jöns Jakob Berzelius identified boron as an element in 1824. The first pure boron was arguably produced by the American chemist W. Weintraub in 1909.[14][15]
Characteristics
Allotropes

Boron is similar to carbon in its capability to form stable covalently bonded molecular networks. Even nominally disordered (amorphous) boron contains regular boron icosahedra which are, however, bonded randomly to each other without long-range order.[16][17] Crystalline boron is a very hard, black material with a high melting point of above 2000 °C. It exists in four major polymorphs: α, β, γ and T. Whereas α, β and T phases are based on B12 icosahedra, the γ-phase can be described as a rocksalt-type arrangement of the icosahedra and B2 atomic pairs.[18] It can be produced by compressing other boron phases to 12–20 GPa and heating to 1500–1800 °C; it remains stable after releasing the temperature and pressure. The T phase is produced at similar pressures, but higher temperatures of 1800–2200 °C. As to the α and β phases, they might both coexist at ambient conditions with the β phase being more stable.[18][19][20] Compressing boron above 160 GPa produces a boron phase with an as yet unknown structure, and this phase is a superconductor at temperatures 6–12 K.[21]
| Boron phase | α | β | γ | T |
|---|---|---|---|---|
| Symmetry | Rhombohedral | Rhombohedral | Orthorhombic | Tetragonal |
| Atoms/unit cell[18] | 12 | ~105 | 28 | |
| Density (g/cm3)[22][23][24][25] | 2.46 | 2.35 | 2.52 | 2.36 |
| Vickers hardness (GPa)[26][27] | 42 | 45 | 50-58 | |
| Bulk modulus (GPa)[27][28] | 185 | 224 | 227 | |
| Bandgap (eV)[27][29] | 2 | 1.6 | 2.1 |
Chemistry of the element
Elemental boron is rare and poorly studied because the material is extremely difficult to prepare. Most studies on "boron" involve samples that contain small amounts of carbon. Chemically, boron behaves more closely to silicon than to aluminium. Crystalline boron is chemically inert and resistant to attack by boiling hydrofluoric or hydrochloric acid. When finely divided, it is attacked slowly by hot concentrated hydrogen peroxide, hot concentrated nitric acid, hot sulfuric acid or hot mixture of sulfuric and chromic acids.[30][14]
The rate of oxidation of boron depends upon the crystallinity, particle size, purity and temperature. Boron does not react with air at room temperature, but at higher temperatures it burns to form boron trioxide:
- 4 B + 3 O2 → 2 B2O3

Boron undergoes halogenation to give trihalides, e.g.:
- 2 B + 3 Br2 → 2 BBr3
These trihalides in practice are usually made from the oxides.
Chemical compounds
In its most familiar compounds, boron has the formal oxidation state III. These include oxides, sulfides, nitrides, and halides.
The trihalides adopt a planar trigonal structure. These compounds are Lewis acids in that they readily form adducts with electron-pair donors, which are called Lewis bases. For example, fluoride (F-) and boron trifluoride (BF3) combined to give the tetrafluoroborate anion, BF4-. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form
Boron is found in nature on Earth entirely as various oxides of B(III), often associated with other elements. The more than one hundred borates all feature boron in oxidation state +3. These mineral resemble silicates in some respect, although boron is often only in tetrahedral coordination, but also trigonal planar. Unlike silicates, the boron minerals never feature boron with coordination number greater than four. A typical motif is exemplified by the tetraborate anions of the common mineral borax, shown at left. The formal negative charge of the tetrahedral borate centers is balanced by metal cations in the minerals, such as the sodium (Na+) in borax.
The boron nitrides are notable for the variety of structures that they adopt. They adopt structures analogous to various allotropes of carbon, including graphite, diamond, and nanotubes. In the diamond-like structure called cubic boron nitride (tradename Borazon), boron atoms exist in the tetrahedral structure of carbons atoms in diamond, but one in every four B-N bonds can be viewed as a coordinate covalent bond, wherein two electrons are donated by the nitrogen atom (which acts as the Lewis base to a bond to the Lewis acidic boron(III) centre. Cubic boron nitride, among other applications, is used as an abrasive, as it has a hardness comparable with diamond (the two substances are able to produce scratches on each other). In the BN compound analogues of graphite, the positively charged boron and negatively charged nitrogen atoms in each plane lie adjacent to the oppositely charged atom in the next plane, consequently graphic and hexagonal boron nitride have very different properties.
Organoboron chemistry
A large number oforganoboron compounds are known and many are useful in organic synthesis. Organoboron(III) compounds are usually tetrahedral or trigonal planar, e.g. tetraphenylborate (B(C6H5)4-) vs triphenylboron (B(C6H5)3). Many are produced from hydroboration, which employs diborane (B2H6).
Compounds of B(I) and B(II)

Boron forms a variety of stable compounds with formal oxidation state less than three. As for many covalent compound, oxidation states are often of little meaning in boron hydrides and metal borides. The halides also form derivatives of B(I) and B(II). BF, isoelectronic with N2, is not isolable in condensed form, but B2F4 and B4Cl4 are well characterized.[31]
Binary metal-boron compounds, the metal borides, feature boron in oxidation state less than III. Illustrative is magnesium diboride (MgB2). In this material, the boron centers are trigonal planar, forming sheets akin to the carbon in graphite. Each boron has a formal -1 charge and magnesium is assigned a formal charge of 2+. In 2001 this material was found to be a high-temperature superconductor.

Other borides find specialized applications as hard materials for cutting tools.
From the structural perspective, the most distinctive chemical compounds of boron are the hydrides. Included in this series are the cluster compounds dodecaborate]] (B12H122-), decaborane (B10H14), and the carboranes such as C2B10H12. Characteristically such compounds feature boron with coordination numbers greater than four.
Isotopes
Boron has two naturally occurring and stable isotopes, 11B (80.1%) and 10B (19.9%). The mass difference results in a wide range of δ11B values, which are defined as a fractional difference between the 11B and 10B and traditionally expressed in parts per thousand, in natural waters ranging from –16 to +59. There are 13 known isotopes of boron, the shortest-lived isotope is 7B which decays through proton emission and alpha decay. It has a half-life of 3.5×10−22 s. Isotopic fractionation of boron is controlled by the exchange reactions of the boron species B(OH)3 and [B(OH)4]−. Boron isotopes are also fractionated during mineral crystallization, during H2O phase changes in hydrothermal systems, and during hydrothermal alteration of rock. The latter effect results in preferential removal of the 10B(OH)4 ion onto clays. It results in solutions enriched in 11B(OH)3 and therefore may be responsible for the large 11B enrichment in seawater relative to both oceanic crust and continental crust; this difference may act as an isotopic signature.[32] The exotic 17B exhibits a nuclear halo, i.e. its radius is appreciably larger than that predicted by the liquid drop model.[33]
Enriched boron (boron-10)
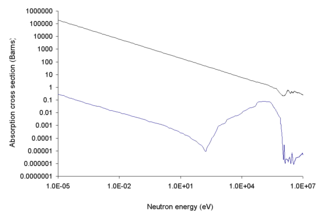
The 10B isotope is good at capturing thermal neutrons. Natural boron is about 20% 10B and 80%11B. The nuclear industry enriches natural boron to nearly pure 10B. The waste product, or depleted boron, is nearly pure 11B. 11B is a candidate as a fuel for aneutronic fusion and is used in the semiconductor industry. Enriched boron or 10B is used in both radiation shielding and in boron neutron capture therapy. In the latter, a compound containing 10B is attached to a muscle near a tumor. The patient is then treated with a relatively low dose of thermal neutrons. This causes energetic and short range alpha radiation from the boron to bombard the tumor.[34][35][36]
In nuclear reactors, 10B is used for reactivity control and in emergency shutdown systems. It can serve either function in the form of borosilicate control rods or as boric acid. In pressurized water reactors, boric acid is added to the reactor coolant when the plant is shut down for refueling. It is then slowly filtered out over many months as fissile material is used up and the fuel becomes less reactive.[37]
In future manned interplanetary spacecraft, 10B has a theoretical role as structural material (as boron fibers or BN nanotube material) which would also serve a special role in the radiation shield. One of the difficulties in dealing with cosmic rays, which are mostly high energy protons, is that some secondary radiation from interaction of cosmic rays and spacecraft materials is high energy spallation neutrons. Such neutrons can be moderated by materials high in light elements such as polyethylene, but the moderated neutrons continue to be a radiation hazard unless actively absorbed in the shielding. Among light elements that absorb thermal neutrons, 6Li and 10B appear as potential spacecraft structural materials which serve both for mechanical reinforcement and radiation protection.[38]
Depleted boron (boron-11)
Cosmic radiation will produce secondary neutrons if it hits spacecraft structures. Those neutrons will be captured in 10B, if it is present in the spacecraft's semiconductors, producing a gamma ray, an alpha particle, and a lithium ion. These resultant decay products may then irradiate nearby semiconductor 'chip' structures, causing data loss (bit flipping, or single event upset). In radiation hardened semiconductor designs, one countermeasure is to use depleted boron which is greatly enriched in 11B and contains almost no 10B. 11B is largely immune to radiation damage. Depleted boron is a by-product of the nuclear industry.[37]
11B is also a candidate as a fuel for aneutronic fusion. When struck by a proton with energy of about 500 keV, it produces three alpha particles and 8.7 MeV of energy. Most other fusion reactions involving hydrogen and helium produce penetrating neutron radiation, which weakens reactor structures and induces long term radioactivity thereby endangering operating personnel. Whereas, the alpha particles from 11B fusion can be turned directly into electric power, and all radiation stops as soon as the reactor is turned off.[39]
NMR spectroscopy
Both 10B and 11B possess nuclear spin. The nuclear spin of 10B is 3 and that of 11B is 3/2. These isotopes are, therefore, of use in nuclear magnetic resonance spectroscopy; and spectrometers specially adapted to detecting the boron-11 nuclei are available commercially. The 10B and 11B nuclei also cause splitting in the resonances of attached nuclei.[40]
Occurrence
See also Category: Borate minerals


Boron is a relatively rare element in the Earth's crust, representing only 0.001%. The worldwide commercial borate deposits are estimated at 10 million tonnes.[41][42] Turkey and the United States are the world's largest producers of boron.[43][44] Turkey has almost 72% of the world’s boron reserves.[45] Boron does not appear on Earth in elemental form but is found combined in borax, boric acid, colemanite, kernite, ulexite and borates. Boric acid is sometimes found in volcanic spring waters.
Ulexite is one of over a hundred borate minerals; it is a fibrous crystal where individual fibers can guide light like optical fibers.[46]
Economically important sources of boron are rasorite (kernite) and tincal (borax ore). They are both found in the Mojave Desert of California, but the largest borax deposits are in Central and Western Turkey including the provinces of Eskişehir, Kütahya and Balıkesir.[47][48][49]
Production
The production of boron compounds does not involve formation of elemental boron, but exploits the convenient availability of borates.
The earliest routes to elemental boron involved reduction of boric oxide with metals such as magnesium or aluminium. However the product is almost always contaminated with metal borides. Pure boron can be prepared by reducing volatile boron halides with hydrogen at high temperatures. Ultrapure boron, for the use in semiconductor industry, is produced by the decomposition of diborane at high temperatures and then further purified with the zone melting or Czochralski processes.[50]
Isotope enrichment
Because of its high neutron cross-section, boron-10 is often used to control fission in nuclear reactors as a neutron-capturing substance.[51] Several industrial-scale enrichment processes have been developed, however only the fractionated vacuum distillation of the dimethyl ether adduct of boron trifluoride (DME-BF3) and column chromatography of borates are being used.[52]
Market trend
Estimated global consumption of boron rose to a record 1.8 million tonnes of B2O3 in 2005, following a period of strong growth in demand from Asia, Europe and North America. Boron mining and refining capacities are considered to be adequate to meet expected levels of growth through the next decade.
The form in which boron is consumed has changed in recent years. The use of ores like colemanite has declined following concerns over arsenic content. Consumers have moved towards the use of refined borates and boric acid that have a lower pollutant content. The average cost of crystalline boron is $5/g.[53]
Increasing demand for boric acid has led a number of producers to invest in additional capacity. Eti Mine Company of Turkey opened a new boric acid plant with the production capacity of 100,000 tonnes per year at Emet in 2003. Rio Tinto Group increased the capacity of its boron plant from 260,000 tonnes per year in 2003 to 310,000 tonnes per year by May 2005, with plans to grow this to 366,000 tonnes per year in 2006. Chinese boron producers have been unable to meet rapidly growing demand for high quality borates. This has led to imports of disodium tetraborate growing by a hundredfold between 2000 and 2005 and boric acid imports increasing by 28% per year over the same period.[54][55]
The rise in global demand has been driven by high growth rates in fiberglass and borosilicate production. A rapid increase in the manufacture of reinforcement-grade fiberglass in Asia with a consequent increase in demand for borates has offset the development of boron-free reinforcement-grade fiberglass in Europe and the USA. The recent rises in energy prices may lead to greater use of insulation-grade fiberglass, with consequent growth in the boron consumption. Roskill Consulting Group forecasts that world demand for boron will grow by 3.4% per year to reach 21 million tonnes by 2010. The highest growth in demand is expected to be in Asia where demand could rise by an average 5.7% per year.[54][56]
Applications
Insulation
The main use of boron compounds is in the form of Sodium tetraborate pentahydrate (Na2B4O7) for making insulating fiberglass and sodium perborate bleach.[57]
Detergents formulations and bleaching agents
Borax is used in laundry products, mainly as a precursor to bleaches. Specifically, sodium perborate serves as a source of active oxygen in many detergents, laundry detergents, cleaning products, and laundry bleaches. It is also present in some tooth bleaching formulas.[57]
Glass and ceramics

Nearly all boron ore extracted from the Earth is destined for refinement into boric acid and sodium tetraborate. In the United States, 70% of the boron is used for the production of glass and ceramics.[58] Borosilicate glass, which is typically 12–15% B2O3, 80% SiO2, and 2% Al2O3, has a low coefficient of thermal expansion giving it a good resistance to thermal shock. Duran and Pyrex are two major brand names for this glass.[59]
Boron filaments are high-strength, lightweight materials that are used chiefly for advanced aerospace structures as a component of composite materials, as well as limited production consumer and sporting goods such as golf clubs and fishing rods.[60][61] The fibers can be produced by chemical vapor deposition of boron on a tungsten filament.[43][62]
Boron fibers and sub-millimeter sized crystalline boron springs are produced by laser-assisted chemical vapor deposition. Translation of the focused laser beam allows to produce even complex helical structures. Such structures show good mechanical properties (elastic modulus 450 GPa, fracture strain 3.7 %, fracture stress 17 GPa) and can be applied as reinforcement of ceramics or in micromechanical systems.[63]
Shielding in nuclear reactors
Boron shielding is used as a control for nuclear reactors, taking advantage of its high cross-section for neutron capture.
Semiconductor industry
Boron is a useful dopant for such semiconductors as silicon, germanium, and silicon carbide. Having one fewer valence electron than the host atom, it donates a hole resulting in p-type conductivity. Traditional method of introducing boron into semiconductors is via its atomic diffusion at high temperatures. This process uses either solid (B2O3), liquid (BBr3), or gaseous boron sources (B2H6 or BF3). However, after 1970s, it was mostly replaced by ion implantation, which relies mostly on BF3 as a boron source.[64] Boron trichloride gas is also an important chemical in semiconductor industry, however not for doping but rather for plasma etching of metals and their oxides.[65] Triethylborane is also injected into vapor deposition reactors as a boron source. Examples are the plasma deposition of boron-containing hard carbon films, silicon nitride-boron nitride films, and for doping of diamond film with boron.[66]
Engineering materials

Boron carbide, a ceramic material which is obtained by decomposing B2O3 with carbon in the electric furnace:
- 2 B2O3 + 7 C → B4C + 6 CO
It is used in tank armor, bulletproof vests, and numerous other structural applications. Its ability to absorb neutrons without forming long lived radionuclides makes the material attractive as an absorbent for neutron radiation arising in nuclear power plants. Nuclear applications of boron carbide include shielding, control rod and shut down pellets. Within control rods, boron carbide is often powdered, to increase its surface area.[67]
High-hardness compounds
| Material | Diamond | cubic-BC2N | cubic-BC5 | cubic-BN | B4C | ReB2 |
|---|---|---|---|---|---|---|
| Vickers hardness (GPa) | 115 | 76 | 71 | 62 | 38 | 22 |
| Fracture toughness (MPa m1/2) | 5.3 | 4.5 | 9.5 | 6.8 | 3.5 |
Several boron compounds are known for their extreme hardness and toughness, including
- Heterodiamond (also called BCN);
- Boron nitride. This material is isoelectronic to carbon. Similar to carbon, it has both hexagonal (soft graphite-like h-BN) and cubic (hard, diamond-like c-BN) forms. h-BN is used as a high temperature component and lubricant. c-BN, also known under commercial name borazon,[70] is a superior abrasive. Its hardness is only slightly smaller, but chemical stability is superior to that of diamond.
- Rhenium diboride can be produced at ambient pressures, but is rather expensive because of rhenium. The hardness of ReB2 exhibits considerable anisotropy because of its hexagonal layered structure. Its value is comparable to that of tungsten carbide, silicon carbide, titanium diboride or zirconium diboride.[71]
- AlMgB14 + TiB2 composites possess high hardness and wear resistance and are used in either bulk form or as coatings for components exposed to high temperatures and wear loads.[72]
Boron carbide and cubic boron nitride powders are widely used as abrasives. Metal borides are used for coating tools through chemical vapor deposition or physical vapor deposition. Implantation of boron ions into metals and alloys, through ion implantation or ion beam deposition, results in a spectacular increase in surface resistance and microhardness. Laser alloying has also been successfully used for the same purpose. These borides are an alternative to diamond coated tools, and their (treated) surfaces have similar properties to those of the bulk boride.[73]
Niche uses

- Boron is a part of neodymium magnet (Nd2Fe14B), which is the strongest type of permanent magnet. They are found in a variety of domestic and professional electromechanical and electronic devices, such as magnetic resonance imaging (MRI), various motors and actuators, computer HDDs, CD and DVD players, mobile phones, timer switches, speakers, etc.[3]
- Starch and casein-based adhesives contain sodium tetraborate decahydrate (Na2B4O7•10 H2O)
- Anti-corrosion systems also contain sodium tetraborate decahydrate.[74]
- Sodium borates are used as a flux for soldering silver and gold and with ammonium chloride for welding ferrous metals.[75] They are also fire retarding additives to plastics and rubber articles.[76]
- Boric acid (also known as orthoboric acid) H3BO3 is used in the production of textile fiberglass and flat panel displays [77] and in many PVAc and PVOH based adhesives.
- Boric acid also has antiseptic, antifungal, and antiviral properties and for this reasons is applied as a water clarifier in swimming pool water treatment.[78] Boric acid is also traditionally used as an insecticide, notably against ants, fleas, and cockroaches.[79]
- Triethylborane is a substance which ignites the JP-7 fuel of the Pratt & Whitney J58 turbojet/ramjet engines powering the Lockheed SR-71 Blackbird.[80] It was also used to ignite the F-1 Engines on the Saturn V Rocket utilized by NASA's Apollo and Skylab programs from 1967 until 1973. Triethylborane is suitable for this because of its pyrophoric properties, especially the fact that it burns with very high temperature.[81] Triethylborane is an industrial initiator in radical reactions, where it is effective even at low temperatures.
Research areas
Magnesium diboride is an important superconducting material with the transition temperature of 39 K. MgB2 wires are produced with the powder-in-tube process and applied in superconducting magnets.[82][83]
Boron compounds show promise in treating arthritis.[84] Because of its distinctive green flame, amorphous boron is used in pyrotechnic flares.[85] It is also used as a melting point depressant in nickel-chromium braze alloys.[86]
Biological role
There is a boron-containing natural antibiotic, boromycin, isolated from streptomyces.[87][88] Boron is an essential plant nutrient, required primarily for maintaining the integrity of cell walls. Conversely, high soil concentrations of > 1.0 ppm can cause marginal and tip necrosis in leaves as well as poor overall growth performance. Levels as low as 0.8 ppm can cause these same symptoms to appear in plants particularly sensitive to boron in the soil. Nearly all plants, even those somewhat tolerant of boron in the soil, will show at least some symptoms of boron toxicity when boron content in the soil is greater than 1.8 ppm. When this content exceeds 2.0 ppm, few plants will perform well and some may not survive. When boron levels in plant tissue exceed 200 ppm symptoms of boron toxicity are likely to appear.[89][90][91]
As an ultratrace element, boron is necessary for the optimal health of rats, although it is necessary in such small amounts that ultrapurified foods and dust filtration of air is necessary to show the effects of boron deficiency, which manifest as poor coat/hair quality. Presumably, boron is necessary to other mammals. No deficiency syndrome in humans has been described. Small amounts of boron occur widely in the diet, and the amounts needed in the diet would, by analogy with rodent studies, be very small. The exact physiological role of boron in the animal kingdom is poorly understood.[92]
Boron occurs in all foods produced from plants. Since 1989 its nutritional value has been argued. It is thought that boron plays several biochemical roles in animals, including humans.[93] The U.S. Department of agriculture conducted an experiment in which postmenopausal women took 3 mg of boron a day. The results showed that supplemental boron reduced excretion of calcium by 44%, and activated estrogen and vitamin D. However, whether these effects were conventionally nutritional, or medicinal, could not be determined. The US National Institutes of Health quotes this source:
- Total daily boron intake in normal human diets ranges from 2.1–4.3 mg boron/day.[94][95]
Analytical quantification
For determination of boron content in food or materials the colorimetric curcumin method is used. Boron has to be transferred to boric acid or borates and on reaction with curcumin in acidic solution, a red colored boron-chelate complex, rosocyanine, is formed.[96]
Health issues
Elemental boron and borates are non-toxic to humans and animals (approximately similar to table salt). The LD50 (dose at which there is 50% mortality) for animals is about 6 g per kg of body weight. Substances with LD50 above 2 g are considered non-toxic. The minimum lethal dose for humans has not been established, but an intake of 4 g/day was reported without incidents, and medical dosages of 20 g of boric acid for neutron capture therapy caused no problems. Fish have survived for 30 min in a saturated boric acid solution and can survive longer in strong borax solutions.[97] Borates are more toxic to insects than to mammals. The boranes and similar gaseous compounds are quite poisonous. As usual, it is not an element that is intrinsically poisonous, but toxicity depends on structure.[9][10]
The boranes (boron hydrogen compounds) are toxic as well as highly flammable and require special care when handling. Sodium borohydride presents a fire hazard due to its reducing nature, and the liberation of hydrogen on contact with acid. Boron halides are corrosive.[98]
Congenital endothelial dystrophy type 2, a rare form of corneal dystrophy, is linked to mutations in SLC4A11 gene that encodes a transporter reportedly regulating the intracellular concentration of boron.[99]
See also
- Allotropes of boron
- Category:Boron compounds
- Boron deficiency
- Boron oxide
- Boron nitride
- Boron neutron capture therapy
- Boronic acid
- Hydroboration-oxidation reaction
- Suzuki coupling
References
- ↑ Fernando, W.T.M.L.; O'Brien, L.C.; Bernath, P.F. (1990). "Fourier Transform Spectroscopy: B4Σ−−X4Σ−". J. Chem. Phys. 93: 8482. doi:10.1063/1.459287. http://bernath.uwaterloo.ca/media/78.pdf.
- ↑ Zhang, K.Q.; Guo, B.; Braun, V.; Dulick, M.; Bernath, P.F. (1995). "Infrared Emission Spectroscopy of BF and AIF". J. Molecular Spectroscopy 170: 82. doi:10.1006/jmsp.1995.1058. http://bernath.uwaterloo.ca/media/125.pdf.
- ↑ 3.0 3.1 Lide, David R. (ed.) (2000). Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics. CRC press. ISBN 0849304814. http://www-d0.fnal.gov/hardware/cal/lvps_info/engineering/elementmagn.pdf.
- ↑ Holcombe Jr., C. E.; Smith, D. D.; Lorc, J. D.; Duerlesen, W. K.; Carpenter; D. A. (1973). "Physical-Chemical Properties of beta-Rhombohedral Boron". High Temp. Sci. 5: 349.
- ↑ 5.0 5.1 "Atomic Weights and Isotopic Compositions for All Elements". National Institute of Standards and Technology. http://physics.nist.gov/cgi-bin/Compositions/stand_alone.pl. Retrieved 2008-09-21.
- ↑ Szegedi, S.; Váradi, M.; Buczkó, Cs. M.; Várnagy, M.; Sztaricskai, T. (1990). "Determination of boron in glass by neutron transmission method". Journal of Radioanalytical and Nuclear Chemistry Letters 146: 177. doi:10.1007/BF02165219.
- ↑ Shipley, Joseph T. (2001). The Origins of English Words: A Discursive Dictionary of Indo-European Roots. JHU Press. ISBN 9780801867842. http://books.google.com/?id=m1UKpE4YEkEC&pg=PA83.
- ↑ "Etymology of Elements". innvista. http://www.innvista.com/science/chemistry/elements/etymolo.htm. Retrieved 2009-06-06.
- ↑ 9.0 9.1 Garrett, Donald E. (1998). Borates: handbook of deposits, processing, properties, and use. Academic Press. pp. 102;385–386. ISBN 0122760603.
- ↑ 10.0 10.1 "Boron". http://mysite.du.edu/~jcalvert/phys/boron.htm. Retrieved 2009-05-05.
- ↑ Davy, H. (1809) "An account of some new analytical researches on the nature of certain bodies, particularly the alkalies, phosphorus, sulphur, carbonaceous matter, and the acids hitherto undecomposed: with some general observations on chemical theory," Philosophical Transactions of the Royal Society of London, vol. 99, pages 33-104.
- ↑ Gay Lussac, J.L. and Thenard, L.J. (1808) "Sur la décomposition et la recomposition de l'acide boracique," Annales de chimie [later: Annales de chemie et de physique], vol. 68, pages 169–174.
- ↑ Weeks, Mary Elvira (1933). "XII. Other Elements Isolated with the Aid of Potassium and Sodium: Beryllium, Boron, Silicon and Aluminum". The Discovery of the Elements. Easton, PA: Journal of Chemical Education. ISBN 0-7661-3872-0.
- ↑ 14.0 14.1 Laubengayer, A. W.; Hurd, D. T.; Newkirk, A. E.; Hoard, J. L. (1943). "Boron. I. Preparation and Properties of Pure Crystalline Boron". Journal of the American Chemical Society 65: 1924–1931. doi:10.1021/ja01250a036.
- ↑ Borchert Dietz, W.; Kolker, H., W. (1970). "Crystal Growth of Beta–Rhombohedrical Boron". Zeitschrift für Angewandte Physik 29: 277.
- ↑ Delaplane, R.G.; Dahlborg, U; Graneli, B; Fischer, P; Lundstrom, T (1988). "A neutron diffraction study of amorphous boron". Journal of Non-Crystalline Solids 104: 249. doi:10.1016/0022-3093(88)90395-X.
- ↑ R.G. Delaplane; Dahlborg, U; Howells, W; Lundstrom, T (1988). "A neutron diffraction study of amorphous boron using a pulsed source". Journal of Non-Crystalline Solids 106: 66. doi:10.1016/0022-3093(88)90229-3.
- ↑ 18.0 18.1 18.2 Oganov A.R., Chen J., Gatti C., Ma Y.-M., Yu T., Liu Z., Glass C.W., Ma Y.-Z., Kurakevych O.O., Solozhenko V.L. (2009). "Ionic high-pressure form of elemental boron". Nature 457 (7231): 863–867. doi:10.1038/nature07736. PMID 19182772. http://mysbfiles.stonybrook.edu/~aoganov/files/Boron-Nature-2009.pdf.
- ↑ van Setten M.J., Uijttewaal M.A., de Wijs G.A., de Groot R.A. (2007). "Thermodynamic stability of boron: The role of defects and zero point motion.". J. Am. Chem. Soc. 129 (9): 2458–2465. doi:10.1021/ja0631246. PMID 17295480.
- ↑ Widom M., Mihalkovic M. (2008). "Symmetry-broken crystal structure of elemental boron at low temperature.". Phys. Rev. B 77 (6): 064113. doi:10.1103/PhysRevB.77.064113.
- ↑ Eremets, M. I.; Struzhkin, VV; Mao, H; Hemley, RJ (2001). "Superconductivity in Boron". Science 293 (5528): 272. doi:10.1126/science.1062286. PMID 11452118.
- ↑ Wentorf Jr, R. H. (1965). "Boron: Another Form". Science 147 (3653): 49–50 (Powder Diffraction File database (CAS number 7440–42–8)). doi:10.1126/science.147.3653.49. PMID 17799779. http://www.sciencemag.org/cgi/content/abstract/147/3653/49.
- ↑ Hoard, J. L.; Sullenger, D. B.; Kennard, C. H. L.; Hughes, R. E. (1970). "The structure analysis of β-rhombohedral boron". J. Solid State Chem. 1: 268–277. doi:10.1016/0022-4596(70)90022-8.
- ↑ Will, G.; Kiefer, B. (2001). "Electron Deformation Density in Rhombohedral a-Boron". Zeitschrift für anorganische und allgemeine Chemie 627: 2100. doi:10.1002/1521-3749(200109)627:9<2100::AID-ZAAC2100>3.0.CO;2-G.
- ↑ Talley, C. P.; LaPlaca, S.; Post, B. (1960). "A new polymorph of boron". Acta Crystallogr. 13: 271. doi:10.1107/S0365110X60000613.
- ↑ Solozhenko, V. L.; Kurakevych, O. O.; Oganov, A. R. (2008). "On the hardness of a new boron phase, orthorhombic γ-B28". Journal of Superhard Materials 30: 428–429. doi:10.3103/S1063457608060117.
- ↑ 27.0 27.1 27.2 Zarechnaya, E. Yu.; Dubrovinsky, L.; Dubrovinskaia, N.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V.; Miyajima, N.; El Goresy, A. et al. (2009). "Superhard Semiconducting Optically Transparent High Pressure Phase of Boron". Phys. Rev. Lett. 102: 185501. doi:10.1103/PhysRevLett.102.185501.
- ↑ Nelmes, R. J.; Loveday, J. S.; Allan, D. R.; Hull, S.; Hamel, G.; Grima, P.; Hull, S. (1993). "Neutron- and x-ray-diffraction measurements of the bulk modulus of boron". Phys. Rev. B 47: 7668. doi:10.1103/PhysRevB.47.7668.
- ↑ ed. Madelung, O. (1983). Landolt-Bornstein, New Series. 17e. Springer-Verlag, Berlin.
- ↑ "WebElements.com – Boron". http://www.webelements.com/boron/. Retrieved 2009-05-05.
- ↑ Greenwood, Norman N.; Earnshaw, Alan. (1997), Chemistry of the Elements (2nd ed.), Oxford: Butterworth-Heinemann, ISBN 0080379419
- ↑ Barth, S. (1997). "Boron isotopic analysis of natural fresh and saline waters by negative thermal ionization mass spectrometry". Chemical Geology 143: 255–261. doi:10.1016/S0009-2541(97)00107-1.
- ↑ Liu, Z. (2003). "Two-body and three-body halo nuclei". Science in China G: Physics Mechanics and Astronomy 46: 441. doi:10.1360/03yw0027.
- ↑ Barth, Rolf F. (2003). "A Critical Assessment of Boron Neutron Capture Therapy: An Overview". Journal of Neuro-Oncology 62 (1): 1–5. doi:10.1023/A:1023262817500.
- ↑ Coderre, Jeffrey A.; Morris, GM (1999). "The Radiation Biology of Boron Neutron Capture Therapy". Radiation Research 151 (1): 1–18. doi:10.2307/3579742. PMID 9973079. http://jstor.org/stable/3579742.
- ↑ Barth, Rolf F.; S; F (15 February 1990). "Boron Neutron Capture Therapy of Cancer". Cancer Research 50 (4): 1061–1070. PMID 2404588. http://cancerres.aacrjournals.org/cgi/content/citation/50/4/1061.
- ↑ 37.0 37.1 Duderstadt, James J.; Hamilton, Louis J. (1976). Nuclear Reactor Analysis. Wiley-Interscience. p. 245. ISBN 0471223638.
- ↑ Doering, R.; Nishi Y. (2007). Handbook of semiconductor manufacturing technology. CRC Press. pp. 31–39. ISBN 1574446754.
- ↑ Nevins, W. M. (1998). "A Review of Confinement Requirements for Advanced Fuels". Journal of Fusion Energy 17 (1): 25–32. doi:10.1023/A:1022513215080.
- ↑ "Boron NMR". BRUKER Biospin. http://rmn.iqfr.csic.es/guide/eNMR/chem/B.html. Retrieved 2009-05-05.
- ↑ Argust, Peter (1998). "Distribution of boron in the environment". Biological Trace Element Research 66 (1–3): 131–143. doi:10.1007/BF02783133. PMID 10050915.
- ↑ Woods, William G. (1994). "An Introduction to Boron: History, Sources, Uses, and Chemistry". Environmental Health Perspectives 102, Supplement 7. http://www.ehponline.org/realfiles/members/1994/Suppl-7/woods-full.html. Retrieved 2008-09-20.
- ↑ 43.0 43.1 Kostick, Dennis S. (2006). "Mineral Yearbook: Boron" (PDF). United States Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/boron/myb1-2006-boron.pdf. Retrieved 2008-09-20.
- ↑ "Mineral Commodity Summaries: Boron" (PDF). United States Geological Survey. 2008. http://minerals.usgs.gov/minerals/pubs/commodity/boron/mcs-2008-boron.pdf. Retrieved 2008-09-20.
- ↑ "Developments in the Economic Sector (of Turkey)". Turkish government. http://www.byegm.gov.tr/YAYINLARIMIZ/kitaplar/turkiye2006/english/302-303.htm. Retrieved 2007-12-21.
- ↑ Simmons, R.; Ahsian, N.; Raven, H. (2007). The Book of Stones: Who They Are and What They Teach. North Atlantic Books. pp. 421–422. ISBN 1556436688.
- ↑ Kistler, R. B. (1994). "Boron and Borates". Industrial Minerals and Rocks (Society of Mining, Metallurgy and Exploration, Inc.): 171–186. http://kisi.deu.edu.tr/cahit.helvaci/Boron.pdf.
- ↑ Zbayolu, G.; Poslu, K. (1992). "Mining and Processing of Borates in Turkey". Mineral Processing and Extractive Metallurgy Review 9 (1–4): 245–254. doi:10.1080/08827509208952709.
- ↑ Kar, Y.; Şen, Nejdet; Demİrbaş, Ayhan (2006). "Boron Minerals in Turkey, Their Application Areas and Importance for the Country's Economy". Minerals & Energy - Raw Materials Report 20 (3–4): 2–10. doi:10.1080/14041040500504293.
- ↑ Berger, L. I. (1996). Semiconductor materials. CRC Press. pp. 37–43. ISBN 0849389127.
- ↑ "Results of the B4C Control Rod Test QUENCH-07". http://bibliothek.fzk.de/zb/berichte/FZKA6746.pdf.
- ↑ "Commissioning of Boron Enrichment Plant". Indira Gandhi Centre for Atomic Research. http://library.igcar.gov.in/html/Contents/IGCNewsletter/nl48/A2.htm. Retrieved 2008-09-21.
- ↑ "Boron Properties". Los Alamos National Laboratory. http://www.rareearth.org/boron_properties.htm. Retrieved 2008-09-18.
- ↑ 54.0 54.1 The Economics of Boron, 11th edition. Roskill Information Services, Ltd.. 2006. ISBN 0862145163.
- ↑ "Raw and Manufactured Materials 2006 Overview". http://www.ceramicindustry.com/Articles/Cover_Story/4b0b7a6ed1cb8010VgnVCM100000f932a8c0____. Retrieved 2009-05-05.
- ↑ "Roskill reports: boron". Roskill. http://www.roskill.com/reports/boron. Retrieved 2009-05-05.
- ↑ 57.0 57.1 Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 0849304857.
- ↑ "Boron: Statistics and Information". USGS. http://minerals.usgs.gov/minerals/pubs/commodity/boron/. Retrieved 2009-05-05.
- ↑ Pfaender, H. G. (1996). Schott guide to glass (2 ed.). Springer. p. 122. ISBN 041262060X.
- ↑ Herring, H. W. (1966). "Selected Mechanical and Physical Properties of Boron Filaments". NASA. http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19660005941_1966005941.pdf. Retrieved 2008-09-20.
- ↑ Layden, G. K. (1973). "Fracture behaviour of boron filaments". Journal of Materials Science 8 (11): 1581–1589. doi:10.1007/BF00754893.
- ↑ Cooke, Theodore F. (1991). "Inorganic Fibers—A Literature Review". Journal of the American Ceramic Society 74 (12): 2959–2978. doi:10.1111/j.1151-2916.1991.tb04289.x.
- ↑ Johansson, S.; Schweitz, Jan-Åke; Westberg, Helena; Boman, Mats (1992). "Microfabrication of three-dimensional boron structures by laser chemical processing". Journal Applied Physics 72: 5956–5963. doi:10.1063/1.351904.
- ↑ May, Gary S.; Spanos, Costas J. (2006). Fundamentals of semiconductor manufacturing and process control. John Wiley and Sons. pp. 51–54. ISBN 0471784060.
- ↑ Sherer, J. Michael (2005). Semiconductor industry: wafer fab exhaust management. CRC Press. pp. 39–60. ISBN 1574447203.
- ↑ Ehrenfried Zschech, Caroline Whelan, Thomas Mikolajick (2005). Materials for information technology: devices, interconnects and packaging. Birkhäuser. p. 44. ISBN 1852339411.
- ↑ Weimer, Alan W. (1997). Carbide, Nitride and Boride Materials Synthesis and Processing. Chapman & Hall (London, New York). ISBN 0-412-54060-6.
- ↑ Solozhenko, V. L.; Kurakevych, Oleksandr O.; Le Godec, Yann; Mezouar, Mohamed; Mezouar, Mohamed (2009). "Ultimate Metastable Solubility of Boron in Diamond: Synthesis of Superhard Diamondlike BC5". Phys. Rev. Lett. 102: 015506. doi:10.1103/PhysRevLett.102.015506.
- ↑ Qin, Jiaqian; He, Duanwei; Wang, Jianghua; Fang, Leiming; Lei, Li; Li, Yongjun; Hu, Juan; Kou, Zili et al. (2008). "Is Rhenium Diboride a Superhard Material?". Advanced Materials 20: 4780. doi:10.1002/adma.200801471.
- ↑ Wentorf, R. H. (1957). "Cubic form of boron nitride". J. Chem Phys. 26: 956. doi:10.1063/1.1745964.
- ↑ Qin, Jiaqian; He, Duanwei; Wang, Jianghua; Fang, Leiming; Lei, Li; Li, Yongjun; Hu, Juan; Kou, Zili et al. (2008). "Is Rhenium Diboride a Superhard Material?". Advanced Materials 20: 4780. doi:10.1002/adma.200801471.
- ↑ Schmidt, Jürgen; Boehling, Marian; Burkhardt, Ulrich; Grin, Yuri (2007). "Preparation of titanium diboride TiB2 by spark plasma sintering at slow heating rate". Science and Technology of Advanced Materials 8: 376. doi:10.1016/j.stam.2007.06.009.
- ↑ Gogotsi, Y. G. and Andrievski, R.A. (1999). Materials Science of Carbides, Nitrides and Borides. Springer. pp. 270–270. ISBN 0792357078.
- ↑ "Borax Decahydrate". http://chemicalland21.com/industrialchem/inorganic/BORAX%20DECAHYDRATE.htm. Retrieved 2009-05-05.
- ↑ Davies, A. C. (1992). The Science and Practice of Welding: Welding science and technology. Cambridge University Press. p. 56. ISBN 052143565X.
- ↑ Horrocks, A.R. and Price, D. (2001). Fire Retardant Materials. Woodhead Publishing Ltd.. p. 55. ISBN 1855734192.
- ↑ Ide, F. (2003). "Information technology and polymers. Flat panel display". Engineering Materials 51: 84. http://sciencelinks.jp/j-east/article/200311/000020031103A0287941.php.
- ↑ "Boric acid". http://chemicalland21.com/industrialchem/inorganic/BORIC%20ACID.htm. Retrieved 2009-05-05.
- ↑ Klotz, J. H.; Moss, JI; Zhao, R; Davis Jr, LR; Patterson, RS (1994). "Oral toxicity of boric acid and other boron compounds to immature cat fleas (Siphonaptera: Pulicidae)". J. Econ. Entomol. 87 (6): 1534–1536. PMID 7836612. http://grande.nal.usda.gov/ibids/index.php?mode2=detail&origin=ibids_references&therow=51171.
- ↑ "Lockheed SR-71 Blackbird". March Field Air Museum. http://www.marchfield.org/sr71a.htm. Retrieved 2009-05-05.
- ↑ A. Young (2008). The Saturn V F-1 Engine: Powering Apollo Into History. Springer. p. 86. ISBN 0387096299.
- ↑ Canfield,, Paul C.; Crabtree, George W. (2003). "Magnesium Diboride: Better Late than Never". Physics Today 56 (3): 34–41. doi:10.1063/1.1570770. http://www.cmp.ameslab.gov/personnel/canfield/pub/pt0303.pdf.
- ↑ Braccini, Valeria; Nardelli, D; Penco, R; Grasso, G (2007). "Development of ex situ processed MgB2 wires and their applications to magnets". Physica C: Superconductivity 456 (1–2): 209–217. doi:10.1016/j.physc.2007.01.030.
- ↑ Travers, Richard L.; Rennie, George; Newnham, Rex (1990). "Boron and Arthritis: The Results of a Double-blind Pilot Study". Journal of Nutritional & Environmental Medicine 1 (2): 127–132. doi:10.3109/13590849009003147.
- ↑ Kosanke, B. J. et al. (2004). Pyrotechnic Chemistry. Journal of Pyrotechnics,. pp. 419. ISBN 9781889526157.
- ↑ Wu, Xiaowei; Chandel, R. S.; Li, Hang (2001). "Evaluation of transient liquid phase bonding between nickel-based superalloys". Journal of Materials Science 36 (6): 1539–1546. doi:10.1023/A:1017513200502.
- ↑ Hütter, R. et al.; Keller-Schierlein, W; Knüsel, F; Prelog, V; Rodgers Jr, GC; Suter, P; Vogel, G; Voser, W et al. (1967). "Stoffwechselprodukte von Mikroorganismen. 57. Mitteilung. Boromycin". Helvetica Chimica Acta 50 (6): 1533–1539. doi:10.1002/hlca.19670500612. PMID 6081908.
- ↑ Dunitz, J. D. et al.; Hawley, DM; Miklos, D; White, DN; Berlin, Y; Marusić, R; Prelog, V (1971). "Structure of boromycin". Helvetica Chimica Acta 54 (6): 1709–1713. doi:10.1002/hlca.19710540624. PMID 5131791.
- ↑ Mahler, R. L.. "Essential Plant Micronutrients. Boron in Idaho". University of Idaho. http://info.ag.uidaho.edu/Resources/PDFs/CIS1085.pdf. Retrieved 2009-05-05.
- ↑ "Functions of Boron in Plant Nutrition" (PDF). U.S. Borax Inc.. http://www.borax.com/agriculture/files/an203.pdf.
- ↑ Blevins, Dale G.; Lukaszewski, KM (1998). "Functions of Boron in Plant Nutrition". Annual Review of Plant Physiology and Plant Molecular Biology 49: 481–500. doi:10.1146/annurev.arplant.49.1.481. PMID 15012243.
- ↑ Nielsen, Forrest H. (1998). "Ultratrace elements in nutrition: Current knowledge and speculation". The Journal of Trace Elements in Experimental Medicine 11 (2–3): 251–274. doi:10.1002/(SICI)1520-670X(1998)11:2/3<251::AID-JTRA15>3.0.CO;2-Q.
- ↑ "Boron". PDRhealth. http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/bor_0040.shtml. Retrieved 2008-09-18.
- ↑ Zook, E. G. (1965). "Total boron". J. Assoc. Off Agric. Chem 48: 850.
- ↑ United States. Environmental Protection Agency. Office of Water, U. S. Environmental Protection Agency Staff (1993). Health advisories for drinking water contaminants: United States Environmental Protection Agency Office of Water health advisories. CRC Press. p. 84. ISBN 087371931X. http://books.google.com/?id=trUdm-GXchIC&pg=PA84.
- ↑ Silverman, L.; Trego, Katherine (1953). "Corrections-Colorimetric Microdetermination of Boron By The Curcumin-Acetone Solution Method". Anal. Chem. 25: 1639. doi:10.1021/ac60083a061.
- ↑ Garrett, Donald E. (1998). Borates. Academic Press. p. 385. ISBN 0122760603. http://books.google.com/?id=imMJJP5T5rsC&pg=PA385.
- ↑ "Environmental Health Criteria 204: Boron". the IPCS. 1998. http://www.inchem.org/documents/ehc/ehc/ehc204.htm. Retrieved 2009-05-05.
- ↑ Vithana, En; Morgan, P; Sundaresan, P; Ebenezer, Nd; Tan, Dt; Mohamed, Md; Anand, S; Khine, Ko; Venkataraman, D; Yong, Vh; Salto-Tellez, M; Venkatraman, A; Guo, K; Hemadevi, B; Srinivasan, M; Prajna, V; Khine, M; Casey, Jr; Inglehearn, Cf; Aung, T (July 2006). "Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary endothelial dystrophy (CHED2).". Nature genetics 38 (7): 755–7. doi:10.1038/ng1824. ISSN 1061-4036. PMID 16767101.
External links
- The Periodic Table of Videos video of Boron at YouTube
- Boron
- WebElements.com – Boron
- National Pollutant Inventory - Boron and compounds
|
|||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
.