Borax
| Borax | |
|---|---|
 |
|
 |
|
|
Sodium tetraborate decahydrate
|
|
| Identifiers | |
| CAS number | 1303-96-4 (decahydrate) |
| Properties | |
| Molecular formula | Na2B4O7·10H2O or Na2[B4O5(OH)4]·8H2O |
| Molar mass | 381.37 |
| Appearance | white solid |
| Density | 1.73 g/cm³ (solid) |
| Melting point |
743 °C[1] |
| Boiling point |
1575 °C |
| Related compounds | |
| Other anions | Sodium aluminate; sodium gallate |
| Other cations | Potassium tetraborate |
| Related compounds | Boric acid, sodium perborate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |

Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.
Borax has a wide variety of uses. It is a component of many detergents, cosmetics, and enamel glazes. It is also used to make buffer solutions in biochemistry, as a fire retardant, as an anti-fungal compound for fiberglass, as an insecticide, as a flux in metallurgy, a texturing agent in cooking, and as a precursor for other boron compounds.
The term borax is used for a number of closely related minerals or chemical compounds that differ in their crystal water content, but usually refers to the decahydrate. Commercially sold borax is usually partially dehydrated.
The word borax is from Persian and originates in the Middle-Persian būrak.
Contents |
Uses
Buffer
Sodium borate is used in biochemical and chemical laboratories to make buffers, e.g. for gel electrophoresis of DNA, such as TBE or the newer SB buffer or BBS (borate buffered saline) in coating procedures. Borate buffers (usually at pH 8) are also used as preferential equilibration solution in DMP-based crosslinking reactions.
Co-complexing
Borax as a source of borate has been used to take advantage of the co-complexing ability of borate with other agents in water to form complex ions with various substances. Borate and a suitable polymer bed are used to chromatograph non-glycosylated hemoglobin differentially from glycosylated hemoglobin (chiefly HbA1c), which is an indicator of long term hyperglycemia in diabetes mellitus. Borate and a proprietary synthetic amino acid, Deselex (from Henkel) have been used to complex water "hardness" cations to make a non-precipitating water "softener". Borate alone does not have a high affinity for "hardness" cations, although it has been used for that purpose.
Flux
A mixture of borax and ammonium chloride is used as a flux when welding iron and steel. It lowers the melting point of the unwanted iron oxide (scale), allowing it to run off. Borax is also used mixed with water as a flux when soldering jewelry metals such as gold or silver. It allows the molten solder to flow evenly over the joint in question. Borax is also a good flux for 'pre-tinning' tungsten with zinc - making the tungsten soft-solderable.[2]
Small-scale mining
Borax is replacing mercury as the preferred method for extracting gold in small-scale mining facilities. The method is called the borax method and is used in the Philippines.[3]
Putty
When a borax-water solution is mixed with PVA glue (wood glue), a rubbery precipitate is formed which is the result of cross-linking in the polymer.[4]
Food additive
Borax, given the E number E285, is used as a food additive in some countries but is banned in the United States. In consequence certain foods, such as caviar, produced for sale in the U.S. contain higher levels of salt to assist preservation.[5] Its use as a cooking ingredient is to add a firm rubbery texture to the food, or as a preservative. In oriental cooking it is mostly used for its texturing properties. In Asia, Borax (Chinese: 硼砂; pinyin: péng shā) or (Chinese: 月石; pinyin: yuè shí) was found to have been added to some Chinese foods like the hand-pulled noodles lamian and some rice noodles like Shahe fen, Kway Teow and Chee Cheong Fun recipes.[6] In Indonesia it is a common, but forbidden, additive to such foods as noodles, meatballs and steamed rice. The country's Directorate of Consumer Protection warns of the risk of liver cancer with high consumption over a period of 5–10 years.[7]
Other uses
- Component of detergents[8]
- Ingredient in enamel glazes
- Component of glass, pottery, and ceramics
- Borax can be used as an additive in ceramic slips and glazes to improve fit on wet, greenware, and bisque.
- Fire retardant
- Anti-fungal compound for fiberglass and cellulose insulation
- Anti-fungal foot soak
- Insecticide to kill ants, cockroaches and fleas
- Precursor for sodium perborate monohydrate that is used in detergents, as well as for boric acid and other borates
- Tackifier ingredient in casein, starch and dextrin based adhesives
- Precursor for Boric acid, a tackifier ingredient in polyvinyl acetate, polyvinyl alcohol based adhesives
- Treatment for thrush in horses' hooves
- Used to make indelible ink for dip pens by dissolving shellac into heated borax
- Curing agent for snake skins
- Curing agent for salmon eggs, for use in sport fishing for salmon
- Swimming pool buffering agent to control the pH
- Neutron absorber, used in nuclear reactors and spent fuel pools to control reactivity and to shut down a nuclear chain reaction
- In agriculture it can be used as a fertilizer to correct boron-poor soils. However, it is required in small amounts; excess can cause injury to plants.[9]
- To clean the brain cavity of a skull for mounting
- To color fires with a green tint[10]
- Was traditionally used to coat dry-cured meats such as hams to protect them from becoming fly-blown during further storage.
- Is found in some commercial vitamin supplements, such as Puritan's Pride Multi-Day.
- For stopping car radiator and engine block leaks[11]
Natural sources

Borax occurs naturally in evaporite deposits produced by the repeated evaporation of seasonal lakes. The most commercially important deposits are found in Turkey; Boron, California; and Searles Lake, California. Also, it has been found at many other locations in the Southwestern United States, the Atacama desert in Chile, and in Tibet and Romania. Borax can also be produced synthetically from other boron compounds.
Toxicity
Borax, sodium tetraborate decahydrate, is not acutely toxic.[12] Its LD50 (median lethal dose) score is tested at 2.66 g/kg in rats:[13] a significant dose of the chemical is needed to cause severe symptoms or death. The lethal dose is not necessarily the same for humans. Simple exposure can cause respiratory and skin irritation; ingestion may cause gastrointestinal distress including nausea, persistent vomiting, abdominal pain, and diarrhea. Effects on the vascular system and brain include headaches and lethargy, but are less frequent. "In severe poisonings, a beefy red skin rash affecting palms, soles, buttocks and scrotum has been described. With severe poisoning, erythematous and exfoliative rash, unconsciousness, respiratory depression, and renal failure." [14]
A reassessment of boric acid/borax by the United States Environmental Protection Agency Office of Pesticide Programs found potential developmental toxicity (especially effects on the testes).[15] Boric acid solutions used as an eye wash or on abraded skin are known to be particularly toxic to infants, especially after repeated use, because of the slow elimination rate.[16]
At a recent European Diagnostics Manufacturing Association (EDMA) meeting, several new additions to the Substance of Very High Concern (SVHC) candidate list in relation to the Registration, Evaluation, Authorisation and restriction of Chemicals Regulations 2007 (REACH) were discussed. The registration and review completed as part of REACH has changed the classification of Sodium Tetraborate CAS 1303-96-4 to: Highly Toxic [17]
Chemistry

The term borax is often used for a number of closely related minerals or chemical compounds that differ in their crystal water content:
- Anhydrous borax (Na2B4O7)
- Borax pentahydrate (Na2B4O7·5H2O)
- Borax decahydrate (Na2B4O7·10H2O)
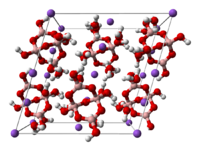
Borax is generally described as Na2B4O7·10H2O. However, it is better formulated as Na2[B4O5(OH)4]·8H2O, since borax contains the [B4O5(OH)4]2− ion. In this structure, there are two four-coordinate boron atoms (two BO4 tetrahedra) and two three-coordinate boron atoms (two BO3 triangles).
Borax is also easily converted to boric acid and other borates, which have many applications. Its reaction with hydrochloric acid to form boric acid is:
- Na2B4O7·10H2O + 2 HCl → 4 B(OH)3 [or H3BO3] + 2 NaCl + 5 H2O
If left exposed to dry air, it slowly loses its water of hydration and becomes the white and chalky mineral tincalconite (Na2B4O7·5H2O).
When borax is added to a flame, it produces a yellow green color.[18] This property has been tried in amateur fireworks, but borax in this use is not popular because its waters of hydration inhibit combustion of compositions and make it an inferior source of the boron which is responsible for most of the green color, and which is overwhelmed by the yellow contributed to the flame by sodium.
However, commercially available borax can be mixed with flammables such as methanol to give the characteristic green flame of boron when ignited, which then slowly gives way to the characteristic yellow-orange flame of the sodium.
See also
- Buffer solution
- Borax bead test
- Sodium borohydride
- Ulexite
- Twenty-Mule-Team Borax
- Francis Marion Smith
- John Veatch
- Boric acid
- List of cleaning agents
References
- ↑ CRC Handbook of Chemistry and Physics 86th edition (2005-2006) section 4 page 88
- ↑ Dodd, J.G. (1966). "Soft soldering to tungsten wire". Am. J. Phys 34 (10): xvi. doi:10.1119/1.1972398
- ↑ "The borax method". Borax replacing mercury in small-scale mining. The Geological Survey of Denmark and Greenland (GEUS). http://www.geus.dk/program-areas/common/int_ssm_fact_sheet_07.pdf. Retrieved 2008-08-02.
- ↑ Parratore, Phil. Wacky Science: A Cookbook for Elementary Teachers. Dubuque, IA: Kendall Hunt. p. 26. ISBN 0787227412.
- ↑ "Caviar glossary". The Caviar Guide a gourmet review of caviars & fish roe. Hanson Ltd, Geneva, Switzerland. http://www.hanscon.ch/caviar_website/glossary.htm. Retrieved 2008-07-07.
- ↑ Chinese Ingredients: Borax Powder, Sep 11, 2005, Chow Hound, Home Cooking
- ↑ Staff writer (2006). "Watch Out For The Food We Consume". Directorate of Consumer Protection, Jakarta, Indonesia. http://pkditjenpdn.depdag.go.id/English/index.php?page=infodtl&InfoID=8&dtl=1. Retrieved 2009-02-10.
- ↑ Record in the Household Products Database of NLM
- ↑ Borax at UC Berkeley
- ↑ http://chemistry.about.com/cs/howtos/a/aa052703a.htm
- ↑ Radweld safety data sheet Retrieved 19-02-2010
- ↑ Borax - toxicity, ecological toxicity and regulatory information
- ↑ Mountain Fresh Dial Bar Soap
- ↑ Borax - toxicity, ecological toxicity and regulatory information
- ↑ Report of the Food Quality Protection Act (FQPA) Tolerance Reassessment Eligibility Decision (TRED) for Boric Acid/Sodium Borate Salts
- ↑ Goodman and Gillman's: The Pharmacological Basis of Therapeutics, 6th edition, chapter on Antiseptics and Disinfectants, page 971
- ↑ http://echa.europa.eu/doc/candidate_list/svhc_supdoc_disodium_tetraborate_anhydrous_publication.pdf
- ↑ Staff. "Creating Flame Colors". The Science Company. http://www.sciencecompany.com/sci-exper/flamecolors.htm. Retrieved November 30, 2008.