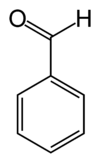
Benzaldehyde
| Benzaldehyde | |
|---|---|
 |
 |
|
Other names
Phenylmethanal
Benzenecarboxaldehyde Benzoic aldehyde |
|
| Identifiers | |
| CAS number | 100-52-7 |
| PubChem | 240 |
| ChemSpider | 235 |
| EC-number | 202-860-4 |
|
SMILES
O=Cc1ccccc1
|
|
|
InChI
InChI=1/C7H6O/c8-6-7-4-2-1-3-5-7/h1-6H
Key: HUMNYLRZRPPJDN-UHFFFAOYAE |
|
| Properties | |
| Molecular formula | C7H6O |
| Molar mass | 106.12 g mol−1 |
| Appearance | colorless liquid |
| Density | 1.0415 g/ml, liquid |
| Melting point |
−26 °C |
| Boiling point |
178.1 °C |
| Solubility in water | 0.6 g/100 ml (20 °C) |
| Viscosity | 1.4 cP (25 °C) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−36.8 kJ/mol |
| Std enthalpy of combustion ΔcH |
−3525.1 kJ/mol |
| Hazards | |
| MSDS | J. T. Baker |
| EU classification | Harmful (Xn) |
| R-phrases | R22 |
| S-phrases | (S2), S24 |
| NFPA 704 |
 2
2
0
|
| Flash point | 63 °C |
| Related compounds | |
| Related aldehydes | anisaldehyde vanillin |
| Related compounds | Benzyl alcohol Benzoic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor. In fact, benzaldehyde is the primary component of bitter almond oil and can be extracted from a number of other natural sources[1].
Contents |
Production
Benzaldehyde can be obtained by many processes. In the 1980s, an estimated 18 million kilograms were produced annually in Japan, Europe, and North America, a level that can be assumed to continue. Currently liquid phase chlorination and oxidation of toluene are the main routes. Numerous other methods have been developed, such as the partial oxidation of benzyl alcohol, alkali hydrolysis of benzal chloride, and the carbonylation of benzene.[2]
Benzaldehyde can be synthesized from cinnamaldehyde obtained from the oil of cinnamon by refluxing in aqueous/alcoholic solution between 90°C and 150°C with a base (most commonly sodium carbonate or bicarbonate) for 5 to 80 hours[3], followed by distillation of the formed benzaldehyde. This reaction also yields acetaldehyde.
Occurrence
Glaciologists LaChapelle and Stillman reported in 1966 that benzaldehyde and N-heptaldehyde inhibit the recrystallization of snow and therefore the formation of depth hoar. This treatment may prevent avalanches caused by unstable depth hoar layers. However, the chemicals are not in widespread use because they damage vegetation and contaminate water supplies.
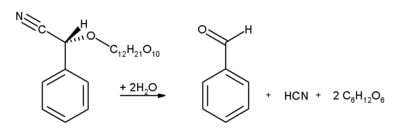
Almonds, apricots, apples and cherry kernels, contain significant amounts of amygdalin. This glycoside breaks up under enzyme catalysis into benzaldehyde, hydrocyanic acid and two molecules of glucose:
Reactions
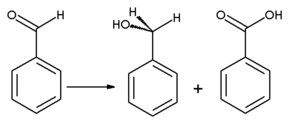
On oxidation, benzaldehyde is converted into the odorless benzoic acid, which is a common impurity in laboratory samples. Benzyl alcohol can be formed from benzaldehyde by means of hydrogenation. Reaction of benzaldehyde with anhydrous sodium acetate and acetic anhydride yields cinnamic acid, while alcoholic potassium cyanide can be used to catalyze the condensation of benzaldehyde to benzoin. Benzaldehyde undergoes disproportionation upon treatment with concentrated alkali (Cannizzaro reaction): one molecule of the aldehyde is reduced to the corresponding alcohol and another molecule is simultaneously oxidized to sodium benzoate.
Uses
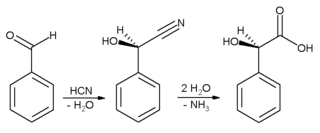
It is commonly employed to confer almond flavor. Benzaldehyde is used chiefly as a precursor to other organic compounds, ranging from pharmaceuticals to plastic additives. The aniline dye malachite green is prepared from benzaldehyde and dimethylaniline. It is a precursor to certain acridine dyes as well. Via aldol condensations, benzaldehyde is converted into derivatives of cinnamaldehyde and styrene. The synthesis of mandelic acid starts from benzaldehyde:
First hydrocyanic acid is added to benzaldehyde, and the resulting nitrile is subsequently hydrolysed to mandelic acid. (The scheme above depicts only one of the two formed enantiomers).
References
- ↑ http://www.freepatentsonline.com/1416128.pdf, United States Patent 1416128 - Process of treating nut kernels to produce food ingredients.
- ↑ Friedrich Brühne and Elaine Wright “Benzaldehyde” in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_463
- ↑ http://www.patentstorm.us/patents/pdfs/patent_id/4617419.html, Process for preparing natural benzaldehyde and acetaldehyde, natural benzaldehyde and acetaldehyde compositions, products produced thereby and organoleptic utilities therefor, Charles Wienes, Middletown; Alan O. Pittet, Atlantic Highlands, both of N.J.