Zidovudine
 |
|
|---|---|
 |
|
| Systematic (IUPAC) name | |
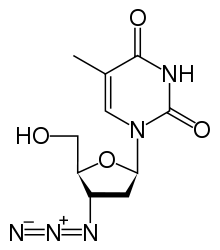
| 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione | |
| Identifiers | |
| CAS number | 30516-87-1 |
| ATC code | J05AF01 |
| PubChem | CID 35370 |
| DrugBank | DB00495 |
| ChemSpider | 32555 |
| Chemical data | |
| Formula | C10H13N5O4 |
| Mol. mass | 267.242 g/mol |
| SMILES | eMolecules & PubChem |
| Pharmacokinetic data | |
| Bioavailability | near complete absoprtion, following first-pass metabolism systemic availability 65% (range 52 to 75%) |
| Protein binding | 30 to 38% |
| Metabolism | Hepatic |
| Half-life | 0.5 to 3 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | B3(AU) C(US) |
| Legal status | ? |
| Routes | Oral |
| |
|
Zidovudine (INN) or azidothymidine (AZT) (also called ZDV) is a nucleoside analog reverse transcriptase inhibitor (NRTI), a type of antiretroviral drug used for the treatment of HIV/AIDs. It is an analog of thymidine.
AZT was the first approved treatment for HIV, sold under the names Retrovir and Retrovis. AZT use was a major breakthrough in AIDS therapy in the 1990s that significantly altered the course of the illness and helped destroy the notion that HIV/AIDS was an instant death sentence. AZT slows HIV spread significantly, but does not stop it entirely. This allows HIV to become AZT resistant over time, and for this reason AZT is usually used in conjunction with other NRTIs and anti-viral drugs. In this form, AZT is used as an ingredient in Combivir and Trizivir, among others. Zidovudine is included in the World Health Organization's "Essential Drugs List", which is a list of minimum medical needs for a basic health care system.[1]
Contents |
History
Development
In the 1950s the theory that most cancers were caused by environmental retroviruses gained currency. It had long been known that most avian cancers were caused by retroviruses, but this knowledge was of limited use in clinical settings due to a lack of drugs that were effective against them. The development of the first anti-retroviral drugs in the 1950s and 60s led to increasing efforts to isolate the expected human analogs of these avian viruses, and then develop suitable drugs to kill them and produce a universal cure for cancer. These efforts eventually culminated in the "War on Cancer" in the 1970s.
AZT was originally studied as one of many attempts to block the action of these oncoviruses. Jerome Horwitz of the Barbara Ann Karmanos Cancer Institute and Wayne State University School of Medicine first synthesized AZT in 1964 under a US National Institutes of Health (NIH) grant.[2][3][4] Development was shelved after it proved insufficiently effective against tumors in mice.[2][5] In 1974, Wolfram Ostertag of the Max Planck Institute in Germany reported that AZT was active against Friend murine leukaemia virus, a retrovirus, in a mouse culture system.[6]
The anti-retroviral approach to develop a "silver bullet" cancer cure ultimately failed. Contrary to early belief, today it is estimated that viruses account for only 10 to 20% of human cancers. However, these efforts advanced the knowledge of cancer considerably, and led to rapid development of many anti-retroviral drugs, which would later prove useful. Zidovudine(AZT) was approved for marketing by the FDA on March 20, 1987.[7]
HIV
In 1984, shortly after the human immunodeficiency virus (HIV) had been confirmed as the cause of AIDS, scientists at the Burroughs-Wellcome Company (now GlaxoSmithKline) began searching for compounds to treat the disease. Burroughs-Wellcome had expertise in viral diseases, led by researchers including Gertrude Elion, David Barry, Phil Furman, Marty St. Clair, Janet Rideout, Sandi Lehman and others. Their research efforts focused on the viral enzyme reverse transcriptase. Reverse transcriptase is an enzyme that retroviruses, including HIV, utilize to replicate themselves. Scientists at Burroughs-Wellcome began to identify and synthesize compounds and developed a screen to test for activity against murine (mouse) retroviruses. One compound, coded "BW A509U", was tested and demonstrated potent activity against mouse viruses.
At the same time, Samuel Broder, Hiroaki Mitsuya, and Robert Yarchoan of the United States National Cancer Institute (NCI) had initiated an independent program to develop therapies for HIV/AIDS. The Burroughs-Wellcome scientists were not working directly with HIV, so the two groups began a collaboration. In February 1985, the NCI scientists showed that BW A509U, one of 11 compounds sent to them by Burroughs-Wellcome, had potent activity against HIV in the test tube. Several months later, Broder, Mitsuya, and Yarchoan started the initial phase 1 clinical trial of AZT at the NCI, in collaboration with scientists from Burroughs-Wellcome and Duke University.[2][8][9][10] This trial suggested that the drug could be safely administered to patients with HIV and that it could increase CD4 counts in HIV-infected patients.
A placebo-controlled randomized trial of AZT was subsequently conducted by Burroughs-Wellcome. The study found that AZT could prolong the life of patients with AIDS.[11] Burroughs-Wellcome filed for a patent for AZT in 1985. The United States Food and Drug Administration (FDA) approved the drug (via the then-new FDA accelerated approval system) for use against HIV, AIDS, and AIDS Related Complex (ARC, a now-defunct medical term for pre-AIDS illness) on March 20, 1987.[7] The time between the first demonstration that AZT was active against HIV in the laboratory and its approval was only 25 months, one of the shortest periods of drug development in recent history.
AZT was subsequently approved as a preventive treatment in 1990. It was initially administered in much higher dosages than today, typically 400 mg every four hours (even at night). However, the unavailability at that time of alternatives to treat AIDS affected the risk/benefit ratio, with the certain toxicity of HIV infection outweighing the risk of drug toxicity. One of AZT's side effects is anemia, a common complaint in early trials.
Current treatment regimens involve lower dosages (e.g., 300 mg) of AZT taken twice a day, almost always as part of highly active antiretroviral therapy (HAART). AZT is combined with other drugs in order to prevent mutation of HIV into an AZT-resistant form.[12][13]
As a solid, AZT forms a hydrogen bonded network of base-paired dimers; its crystal structure was reported in 1988.[14]
Prophylaxis
AZT may be used in combination with other antiretroviral medications to substantially reduce the risk of HIV infection following a significant exposure to the virus (such as a needle-stick injury involving blood or body fluids from an individual known to be infected with HIV).[15]
AZT is also recommended as part of a regimen to prevent mother-to-child transmission of HIV during pregnancy, labor, and delivery.[16] With no treatment, approximately 25% of infants whose mothers are infected with HIV will become infected. AZT has been shown to reduce this risk to approximately 8% when given in a three-part regimen during pregnancy, delivery and to the infant for 6 weeks after birth.[17] During the period from 1994 to 1999 when this was the primary form of prevention of mother-to-child HIV transmission, AZT prophylaxis prevented more than 1000 infant HIV infections.[18] Use of appropriate combinations of antiretroviral medications, cesarean section and avoidance of breast feeding can further reduce mother-child transmission of HIV to 1–2%.
Side effects
Common side effects of AZT include nausea, headache, changes in body fat, and discoloration of fingernails and toenails. More severe side effects include anemia and bone marrow suppression, which can be overcome using erythropoietin or darbepoetin treatments. These unwanted side effects might be caused by the sensitivity of the γ-DNA polymerase in the cell mitochondria. Drugs that inhibit hepatic glucuronidation, such as indomethacin, acetylsalicylic acid (Aspirin) and trimethoprim, decrease the elimination rate and increase the toxicity.[19]
Viral resistance
AZT does not destroy the HIV infection, but only delays the progression of the disease and the replication of virus, even at very high doses. During prolonged AZT treatment, HIV has the ability to gain an increased resistance to AZT by mutation of its reverse transcriptase. To slow the development of resistance, physicians generally recommend that AZT be given in combination with another reverse transcriptase inhibitor and an antiretroviral from another group, such as a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor, this type of therapy is known as HAART (Highly Active Anti Retroviral Therapy). AZT has been shown to work additively or synergistically with many antiviral agents such as acyclovir and interferon; however, ribavirin decreases the antiviral effect of AZT.
Mode of action
Like other reverse transcriptase inhibitors, AZT works by inhibiting the action of reverse transcriptase, the enzyme that HIV uses to make a DNA copy of its RNA. Reverse transcription is necessary for production of the viral double-stranded DNA, which is subsequently integrated into the genetic material of the infected cell (where it is called a provirus).[20][8][10]
The azido group increases the lipophilic nature of AZT, allowing it to cross cell membranes easily by diffusion and thereby also to cross the blood-brain barrier. Cellular enzymes convert AZT into the effective 5'-triphosphate form. Studies have shown that the termination of the formed DNA chains is the specific factor in the inhibitory effect.
The triphosphate form also inhibits DNA polymerase used by human cells to undergo cell division[21] but is far more effective at inhibiting reverse transcriptase than it is at inhibiting DNA polymerase alpha making it much better at inhibiting HIV replication than interfering with human cell division.[22][8] The cellular DNA polymerase used by mitochondria to replicate is more sensitive to the inhibitory effects of AZT, accounting for its toxic effects on cardiac and skeletal muscles, causing myositis.[23][24][25][26][9]
Patent issues
The patents on AZT have been the target of some controversy. In 1991, Public Citizen filed a lawsuit claiming that the patents were invalid. Subsequently, Barr Laboraties and Novopharm Ltd. also challenged the patent, in part based on the assertion that NCI scientists Samuel Broder, Hiroaki Mitsuya, and Robert Yarchoan should have been named as inventors, and those two companies applied to the FDA to sell AZT as a generic drug. In response, Burroughs Wellcome Co. filed a lawsuit against the two companies. The United States Court of Appeals for the Federal Circuit ruled in 1992 in favor of Burroughs Wellcome, claiming that even though they had never tested it against HIV, they had conceived of it working before they sent it to the NCI scientists.[27] In 2002, another lawsuit was filed over the patent by the AIDS Healthcare Foundation.
However, the patent expired in 2005 (placing AZT in the public domain), allowing other drug companies to manufacture and market generic AZT without having to pay GlaxoSmithKline any royalties. The U.S. FDA has since approved four generic forms of AZT for sale in the U.S.
References
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. March 2005. http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf. Retrieved 2006-03-12.
- ↑ 2.0 2.1 2.2 PMID 20018391 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Horowitz, JP; Chua J; Noel MJ (1964). "The monomesylates of 1-(2-deoxy-bd-lyxofuranosyl) thymines". Org. Chem. Ser. Monogr 29: 2076-9. doi:10.1021/jo01030a546.
- ↑ Detours V; Henry D (writers/directors). (2002). I am alive today (history of an AIDS drug). [Film]. ADR Productions/Good & Bad News.
- ↑ "A Failure Led to Drug Against AIDS". The New York Times. 1986-09-20. http://query.nytimes.com/gst/fullpage.html?sec=health&res=9A0DE4D81F3CF933A1575AC0A960948260. Retrieved 2010-06-30.
- ↑ PMID 4531031 (PubMed)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 7.0 7.1 Cimons, Marlene (21 March 1987). "U.S. Approves Sale of AZT to AIDS Patients". Los Angeles Times: pp. 1.
- ↑ 8.0 8.1 8.2 Mitsuya H, Weinhold K, Furman P, St Clair M, Lehrman S, Gallo R, Bolognesi D, Barry D, Broder S (1985). "3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro.". Proc Natl Acad Sci USA 82 (20): 7096–100. doi:10.1073/pnas.82.20.7096. PMID 2413459.
- ↑ 9.0 9.1 Yarchoan R, Mitsuya H, Myers C, Broder S (1989). "Clinical pharmacology of 3'-azido-2',3'-dideoxythymidine (zidovudine) and related dideoxynucleosides.". N Engl J Med 321 (11): 726–38. PMID 2671731.
- ↑ 10.0 10.1 Yarchoan R, Klecker R, Weinhold K, Markham P, Lyerly H, Durack D, Gelmann E, Lehrman S, Blum R, Barry D (1986). "Administration of 3'-azido-3'-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex.". Lancet 1 (8481): 575–80. doi:10.1016/S0140-6736(86)92808-4. PMID 2869302.
- ↑ Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT, et al. (1987). "The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial.". N Engl J Med 317 (4): 185–91. PMID 3299089.
- ↑ De Clercq E (1994). "HIV resistance to reverse transcriptase inhibitors.". Biochem Pharmacol 47 (2): 155–69. doi:10.1016/0006-2952(94)90001-9. PMID 7508227.
- ↑ Yarchoan R, Mitsuya H, Broder S (1988). "AIDS therapies.". Sci Am 259 (4): 110–9. doi:10.1038/scientificamerican1088-110. PMID 3072667.
- ↑ Dyer I, Low JN, Tollin P, Wilson HR, Howie RA (April 1988). "Structure of 3'-azido-3'-deoxythymidine, AZT". Acta Crystallogr C 44 ( Pt 4): 767–9. PMID 3271074.
- ↑ "Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV". http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Retrieved 2006-03-29.
- ↑ "Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health". http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. Retrieved 2006-03-29.
- ↑ Connor E, Sperling R, Gelber R, Kiselev P, Scott G, O'Sullivan M, VanDyke R, Bey M, Shearer W, Jacobson R (1994). "Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group.". N Engl J Med 331 (18): 1173–80. doi:10.1056/NEJM199411033311801. PMID 7935654.
- ↑ Walensky RP, Paltiel AD, Losina E, et al. (July 2006). "The survival benefits of AIDS treatment in the United States". J. Infect. Dis. 194 (1): 11–9. doi:10.1086/505147. PMID 16741877.
- ↑ "ZIDOVUDINE (AZT) - ORAL (Retrovir) side effects, medical uses, and drug interactions". MedicineNet. http://www.medicinenet.com/zidovudine_azt-oral/article.htm. Retrieved 2006-01-09.
- ↑ Mitsuya H, Yarchoan R, Broder S (1990). "Molecular targets for AIDS therapy.". Science 249 (4976): 1533–44. doi:10.1126/science.1699273. PMID 1699273.
- ↑ Plessinger M, Miller R (1999). "Effects of zidovudine (AZT) and dideoxyinosine (ddI) on human trophoblast cells.". Reprod Toxicol 13 (6): 537–46. doi:10.1016/S0890-6238(99)00052-0. PMID 10613402.
- ↑ Furman P, Fyfe J, St Clair M, Weinhold K, Rideout J, Freeman G, Lehrman S, Bolognesi D, Broder S, Mitsuya H (1986). "Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase.". Proc Natl Acad Sci USA 83 (21): 8333–7. doi:10.1073/pnas.83.21.8333. PMID 2430286.
- ↑ Collins M, Sondel N, Cesar D, Hellerstein M (2004). "Effect of nucleoside reverse transcriptase inhibitors on mitochondrial DNA synthesis in rats and humans.". J Acquir Immune Defic Syndr 37 (1): 1132–9. doi:10.1097/01.qai.0000131585.77530.64. PMID 15319672.
- ↑ Parker W, White E, Shaddix S, Ross L, Buckheit R, Germany J, Secrist J, Vince R, Shannon W (1991). "Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase and human DNA polymerases alpha, beta, and gamma by the 5'-triphosphates of carbovir, 3'-azido-3'-deoxythymidine, 2',3'-dideoxyguanosine and 3'-deoxythymidine. A novel RNA template for the evaluation of antiretroviral drugs.". J Biol Chem 266 (3): 1754–62. PMID 1703154.
- ↑ Rang H.P., Dale M.M., Ritter J.M. (1995). Pharmacology (3rd ed.). Pearson Professional Ltd. ISBN 0443059748.
- ↑ Balzarini J, Naesens L, Aquaro S, Knispel T, Perno C, De Clercq E, Meier C (1 December 1999). "Intracellular metabolism of CycloSaligenyl 3'-azido-2', 3'-dideoxythymidine monophosphate, a prodrug of 3'-azido-2', 3'-dideoxythymidine (zidovudine).". Mol Pharmacol 56 (6): 1354–61. PMID 10570065. http://molpharm.aspetjournals.org/cgi/content/full/56/6/1354.
- ↑ US Court of Appeals for the Federal Circuit. "Burroughs Wellcome Co. v. Barr Laboratories, 40 F.3d 1223 (Fed. Cir. 1994)". University of Houston -- Health Law and Policy Institute. http://www.law.uh.edu/healthlaw/law/FederalMaterials/FederalCases/BurroughsWellcomevBarrLaboratories.htm. Retrieved 2007-02-28.
External links
|
|||||||||||||||||||||||||||||||||||||||||||||||||||