Auxin
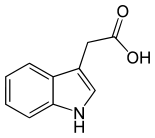
Auxins are a class of plant growth substance and morphogens (often called phytohormone or plant hormone). Auxins have an essential role in coordination of many growth and behavioral processes in the plant life cycle. Auxins and their role in plant growth were first revealed by the Dutch scientist Frits Went.[1].
Contents |
Overview
Auxins derive their name from the Greek word αυξανω ("auxano" -- "I grow/increase"). They were the first of the major plant hormones to be discovered.
Their patterns of active transport through the plant are complex. They typically act in concert with, or in opposition to other plant hormones. For example, the ratio of auxin to cytokinin in certain plant tissues determines initiation of root versus shoot buds. Thus a plant can (as a whole) react to external conditions and adjust to them, without requiring a nervous system. On the molecular level, auxins have an aromatic ring and a carboxylic acid group (Taiz and Zeiger, 1998).
The most important member of the auxin family is indole-3-acetic acid (IAA). It generates the majority of auxin effects in intact plants, and is the most potent native auxin. However, molecules of IAA are chemically labile in aqueous solution, so IAA is not used commercially as a plant growth regulator.
- Naturally-occurring auxins include 4-chloro-indoleacetic acid, phenylacetic acid (PAA) and indole-3-butyric acid (IBA).
- Synthetic auxin analogs include 1-naphthaleneacetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D), and others.
 indole-3-acetic acid (IAA) |
 Indole-3-butyric acid (IBA) |
 4-chloroindole-3-acetic acid (4-CI-IAA) |
 2-phenylacetic acid (PAA) |
 2,4-Dichlorophenoxyacetic acid (2,4-D) |
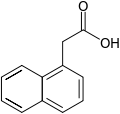 α-Naphthalene acetic acid (α-NAA) |
 2-Methoxy-3,6-dichlorobenzoic acid (dicamba) |
 4-Amino-3,5,6-trichloropicolinic acid (tordon or picloram) |
isobutyric_acid.png) α-(p-Chlorophenoxy)isobutyric acid (PCIB, an antiauxin) |
Auxins are often used to promote initiation of adventitious roots and are the active ingredient of the commercial preparations used in horticulture to root stem cuttings. They can also be used to promote uniform flowering, to promote fruit set, and to prevent premature fruit drop.
Used in high doses, auxin stimulates the production of ethylene. Excess ethylene can inhibit elongation growth, cause leaves to fall (leaf abscission), and even kill the plant. Some synthetic auxins such as 2,4-D and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) have been used as herbicides.
Broad-leaf plants (dicots) such as dandelions are much more susceptible to auxins than narrow-leaf plants (monocots) like grass and cereal crops. These synthetic auxins were the active agents in Agent Orange, a defoliant used extensively by American forces in the Vietnam War.
Hormonal activity
Auxins coordinate development at all levels in plants, from the cellular level through organs and ultimately the whole plant.
Molecular mechanisms
Auxins directly stimulate or inhibit the expression of specific genes.[2] Auxin induces transcription by targeting for degradation members of the Aux/IAA family of transcriptional repressor proteins, The degradation of the Aux/IAAs leads to the derepression of Auxin Respose Factors ARF-mediated transcription. Aux/IAAs are targeted for degradation by ubiquitination, catalysed by an SCF-type ubiquitin-protein ligase.
In 2005, it was demonstrated that the F-box protein TIR1, which is part of the ubiquitin ligase complex SCFTIR1, is an auxin receptor. Upon binding of auxin, TIR1 recruits specific transcriptional repressors (the Aux/IAA repressors) for ubiquitination by the SCF complex. This marking process leads to the degradation of the repressors by the proteasome, alleviating repression and leading to expression of specific genes in response to auxins (reviewed in[3]).
Another protein called ABP1 (Auxin Binding Protein 1) is a putative receptor, but its role is unclear. Electrophysiological experiments with protoplasts and anti-ABP1 antibodies suggest that ABP1 may have a function at the plasma membrane.
On a cellular level
On the cellular level, auxin is essential for cell growth, affecting both cell division and cellular expansion. Depending on the specific tissue, auxin may promote axial elongation (as in shoots), lateral expansion (as in root swelling), or isodiametric expansion (as in fruit growth). In some cases (coleoptile growth) auxin-promoted cellular expansion occurs in the absence of cell division. In other cases, auxin-promoted cell division and cell expansion may be closely sequenced within the same tissue (root initiation, fruit growth). In a living plant it appears that auxins and other plant hormones nearly always interact to determine patterns of plant development.
According to the acid growth hypothesis for auxin action, auxins may directly stimulate the early phases of cell elongation by causing responsive cells to actively transport hydrogen ions out of the cell, thus lowering the pH around cells. This acidification of the cell wall region activates wall-loosening proteins known as expansins, which allow slippage of cellulose microfibrils in the cell wall, making the cell wall less rigid. When the cell wall is loosened by the action of auxins, this now-less-rigid wall is expanded by cell turgor pressure, which presses against the cell wall.
However, the acid growth hypothesis does not by itself account for the increased synthesis and transport of cell wall precursors and secretory activity in the Golgi system that accompany and sustain auxin-promoted cell expansion.
Organ patterns
Growth and division of plant cells together result in growth of tissue, and specific tissue growth contributes to the development of plant organs. Growth of cells contributes to the plant's size, but uneven localized growth produces bending, turning and directionalization of organs- for example, stems turning toward light sources (phototropism), roots growing in response to gravity (gravitropism), and other tropisms.
Organization of the plant
As auxins contribute to organ shaping, they are also fundamentally required for proper development of the plant itself. Without hormonal regulation and organization, plants would be merely proliferating heaps of similar cells. Auxin employment begins in the embryo of the plant, where directional distribution of auxin ushers in subsequent growth and development of primary growth poles, then forms buds of future organs. Throughout the plant's life, auxin helps the plant maintain the polarity of growth and recognize where it has its branches (or any organ) connected.
An important principle of plant organization based upon auxin distribution is apical dominance, which means that the auxin produced by the apical bud (or growing tip) diffuses downwards and inhibits the development of ulterior lateral bud growth, which would otherwise compete with the apical tip for light and nutrients. Removing the apical tip and its suppressive hormone allows the lower dormant lateral buds to develop, and the buds between the leaf stalk and stem produce new shoots which compete to become the lead growth. This behavior is used in pruning by horticulturists.
Uneven distribution of auxin: To cause growth in the required domains, it is necessary that auxins be active preferentially in them. Auxins are not synthesized everywhere, but each cell retains the potential ability to do so, and only under specific conditions will auxin synthesis be activated. For that purpose, not only do auxins have to be translocated toward those sites where they are needed but there has to be an established mechanism to detect those sites. Translocation is driven throughout the plant body primarily from peaks of shoots to peaks of roots. For long distances, relocation occurs via the stream of fluid in phloem vessels, but, for short-distance transport, a unique system of coordinated polar transport directly from cell to cell is exploited. This process of polar auxin transport is directional and very strictly regulated. It is based in uneven distribution of auxin efflux carriers on the plasma membrane, which send auxins in the proper direction.
A 2006 study showed plant-specific pin-formed (PIN) proteins are vital in transporting auxin.[4]
The regulation of PIN protein localisation in a cell determines the directional transport of auxin to create the peaks of auxin, or auxin maxima. These auxin maxima help organise the development of the root and shoot.[5].[6] . Surrounding auxin maxima are cells with low auxin troughs, or auxin minima. In the Arabidopsis fruit auxin minima have been shown to be important for tissue development[7].
Locations
- In shoot (and root) meristematic tissue
- In young leaves
- In mature leaves in very tiny amounts
- In mature root cells in even smaller amounts
- Transported throughout the plant more prominently downward from the shoot apices
Effects

Auxin stimulates cell elongation by stimulating wall loosening factors, such as elastins, to loosen cell walls. The effect is stronger if gibberellins are also present. Auxin also stimulates cell division if cytokinins are present. When auxin and cytokinin are applied to callus, rooting can be generated if the auxin concentration is higher than cytokinin concentration. Xylem tissues can be generated when the auxin concentration is equal to the cytokinins.
Auxin participates in phototropism, geotropism, hydrotropism and other developmental changes. The uneven distribution of auxin, due to environmental cues such as unidirectional light or gravity force, results in uneven plant tissue growth.
Auxin also induces sugar and mineral accumulation at the site of application.
Wounding response
Auxin induces the formation and organization of phloem and xylem. When the plant is wounded, the auxin may induce the Cell differentiation and regeneration of the vascular tissues.
Root growth and development
Auxin induces new root formation by breaking root apical dominance induced by cytokinins. In horticulture, auxins, especially NAA and IBA, are commonly applied to stimulate root growth when taking cuttings of plants. However, high concentrations of auxin inhibit root elongation and instead enhance adventitious root formation. Removal of the root tip can lead to inhibition of secondary root formation.
Apical dominance
Auxin induces shoot apical dominance; the axillary buds are inhibited by auxin. When the apex of the plant is removed, the inhibitory effect is removed and the growth of lateral buds is enhanced as a high concentration of auxin directly stimulates ethylene synthesis in lateral buds causes inhibition of its growth and potentiation of apical dominance.
Ethylene biosynthesis
In low concentrations, auxin can inhibit ethylene formation and transport of precursor in plants; however, high concentrations of auxin can induce the synthesis of ethylene. Therefore, the high concentration can induce femaleness of flowers in some species.
Auxin inhibits abscission prior to formation of abscission layer and thus inhibits senescence of leaves.
Fruit growth and development
Auxin delays fruit senescence.
Auxin is required for fruit growth and development. When seeds are removed from strawberries, fruit growth is stopped; exogenous auxin stimulates the growth in seed removed fruits. For fruit with unfertilized seeds, exogenous auxin results in parthenocarpy ("virgin-fruit" growth).
Auxin is important for the correct development of fruit. Fruits form abnormal morphologies when auxin transport is disturbed.[8]. In Arabidopsis fruits auxin controls the release of seeds from the fruit (pod). The valve margins are a specialised tissue in pods that regulates when pod will open (dehiscence). Auxin must be removed from the valve margin cells to allow the valve margins to form. This process requires modification of the auxin transporters.[7].
Flowering
Auxin plays a minor role in the initiation of flowering. It can delay the senescence of flowers in low concentrations.
Herbicide manufacture
The defoliant Agent Orange was a mix of 2,4-D and 2,4,5-T. The compound 2,4-D is still in use and is thought to be safe, but 2,4,5-T was more or less banned by the EPA in 1979. The dioxin TCDD is an unavoidable contaminant produced in the manufacture of 2,4,5-T. As a result of the integral dioxin contamination, 2,4,5-T has been implicated in leukaemia, miscarriages, birth defects, liver damage, and other diseases. Agent Orange was sprayed in Vietnam as a defoliant to deny ground cover to the Vietnamese army.
See also
- Herbicide
- Pruning fruit trees
- Fusicoccin
References
- ↑ Auxins
- ↑ Hardtke CS. Transcriptional auxin-brassinosteroid crosstalk: who's talking? Bioessays. 2007 Nov;29(11):1115-23. PMID: 17935219
- ↑ Delker C. et al., Auxin dynamics: the dazzling complexity of a small molecule’s message. Planta. Apr. 2008, 227(5) 929-941
- ↑ Petrášek et al. PIN Proteins Perform a Rate-Limiting Function in Cellular Auxin Efflux. Science 12 May 2006:312, 914-918 (2006)
- ↑ Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root Cell. 1999 Nov 24;99(5):463-72
- ↑ Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005 Nov 8;15(21):1899-911.
- ↑ 7.0 7.1 Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galván-Ampudia CS, Offringa R, Friml J, Yanofsky MF, Østergaard L. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature. 2009 May 28;459(7246):583-6.
- ↑ Jennifer L. Nemhauser*, Lewis J. Feldman and Patricia C. Zambryski*. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127, 3877-3888 (2000)
- Plant Physiology Online - Chapter 19: Auxin: The Growth Hormone
- Plant Physiology
- Taiz, L. & Zeiger, E. (1998). Plant Physiology. 2nd edition. Massachusetts: Sinauer Associates, Inc. 792 p.
|
||||||||