Mimicry
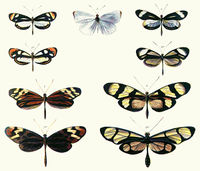
Mimicry[1] is the similarity of one species to another which protects one or both.[2] This similarity can be in appearance, behaviour, sound, scent and even location, with the mimics found in similar places to their models.[3]
Mimicry occurs when a group of organisms,[4] the mimics, evolve to share common perceived characteristics with another group, the models.[5] The evolution is driven by the selective action of a signal-receiver, or dupe.[6] For example, birds that use sight to identify palatable insects (the mimics), whilst avoiding the noxious models.
Collectively, this situation is known as a mimicry complex.[6] The model is usually another species except in cases of automimicry. The signal-receiver is typically another intermediate organism like the common predator of two species, but may actually be the model itself, such as a moth resembling its spider predator.[7] As an interaction, mimicry is in most cases advantageous to the mimic and harmful to the receiver, but may increase, reduce or have no effect on the fitness of the model depending on the situation. Models themselves are difficult to define in some cases, for example eye spots may not bear resemblance to any specific organism's eyes, and camouflage often cannot be attributed to a particular model.

Camouflage, in which a species resembles its surroundings, is essentially a form of visual mimicry. In between camouflage and mimicry is mimesis, in which the mimic takes on the properties of a specific object or organism, but one to which the dupe is indifferent.[8] The lack of a true distinction between the two phenomena can be seen in animals that resemble twigs, bark, leaves or flowers, in that they are often classified as camouflaged (a plant constitutes its "surroundings"), but are sometimes classified as mimics (a plant is also an organism).[3]p51 Crypsis is a broader concept which encompasses all forms of avoiding detection, such as mimicry, camouflage, hiding etc.[9]
Though visual mimicry is most obvious to humans, other senses such as olfaction (smell) or hearing may be involved, and more than one type of signal may be employed.[3] Mimicry may involve morphology, behavior, and other properties. In any case, the signal always functions to deceive the receiver by preventing it from correctly identifying the mimic. In evolutionary terms, this phenomenon is a form of co-evolution usually involving an evolutionary arms race.[9]p161 It should not be confused with convergent evolution, which occurs when species come to resemble one another independently by adapting to similar lifestyles.
Mimics may have different models for different life cycle stages, or they may be polymorphic, with different individuals imitating different models. Models themselves may have more than one mimic, though frequency dependent selection favors mimicry where models outnumber mimics. Models tend to be relatively closely related organisms,[10] but mimicry of vastly different species is also known. Most known mimics are insects,[3] though many other animal mimics including mammals are known. Plants and fungi may also be mimics, though less research has been carried out in this area.[11][12][13]
Contents |
Etymology
Use of the word mimicry dates back to 1637. It is derived from the Greek term mimetikos, "imitative," in turn from mimetos, the verbal adjective of mimeisthai, "to imitate." Originally used to describe people, it was only applied to other forms of life after 1851.[14]
Classification
Many types of mimicry have been described. An overview of each follows, highlighting the similarities and differences between the various forms. Classification is often based on function with respect to the mimic (e.g. avoiding harm), though other parameters can also be used, and multidimensional classifications are required to understand the full picture. For this reason, some cases may belong to more than one class, e.g. automimicry and aggressive mimicry are not mutually exclusive, as one describes the species relationship between model and mimic, while the other describes the function for the mimic (obtaining food).
Defensive

Defensive or protective mimicry takes place when organisms are able to avoid an encounter that would be harmful to them by deceiving an enemy into treating them as something else. Four such cases are discussed here, the first three of which entail mimicry of an aposematic, harmful organism: Batesian mimicry, where a harmless mimic poses as harmful; Müllerian mimicry, where two harmful species share similar perceived characteristics; and Mertensian mimicry, where a deadly mimic resembles a less harmful but lesson-teaching model. Finally, Vavilovian mimicry, where weeds resemble crops, is discussed.
Batesian


In Batesian mimicry the mimic shares signals similar to the model, but does not have the attribute that makes it unprofitable to predators (e.g. unpalatability). In other words, a Batesian mimic is a sheep in wolf's clothing. It is named after Henry Walter Bates, an English naturalist whose work on butterflies in the Amazon rainforest (including Naturalist on the River Amazons) was pioneering in this field of study.[16][17] Mimics are less likely to be found out when in low proportion to their model, a phenomenon known as negative frequency dependent selection which applies in most other forms of mimicry as well. This is not the case in Müllerian mimicry however, which is described next. Examples:
- Lepidoptera
- The Ash Borer (Podosesia syringae), a moth of the Clearwing family (Sesiidae), is a Batesian mimic of the Common wasp because it resembles the wasp, but is not capable of stinging. A predator that has learned to avoid the wasp would similarly avoid the Ash Borer.
- Plain Tiger (Danaus chrysippus) – an unpalatable model with a number of mimics.
- Common Crow (Euploea core) – an unpalatable model with a number of mimics. See also under Müllerian mimicry below.
- Consul fabius and Eresia eunice imitate unpalatable Heliconius butterflies such as H. ismenius.[18]
- Several palatable butterflies resemble different species from the highly noxious papilionine genus Battus.[18]
- Several palatable moths produce ultrasonic click calls to mimic the unpalatable tiger moths.[19]
- The False Cobra (Malpolon moilensis) is a mildly venomous but harmless colubrid snake which mimics the characteristic "hood" of an Indian cobra's threat display. The Eastern Hognose Snake (Heterodon platirhinos) similarly mimics the threat display of venomous snakes.
- The milk snake resembles the deadly coral snake.
- Vespid wasps bear several harmless mimics including moths, beetles and hoverflies.
- Octopuses of the genus Thaumoctopus (the Mimic Octopus) are able to intentionally alter their body shape and color so that they resemble dangerous sea snakes or lionfish.[20]
Müllerian
Müllerian mimicry describes a situation where two or more species have very similar warning or aposematic signals and both share genuine anti-predation attributes (e.g. being unpalatable). At first Bates could not explain why this should be so; if both were harmful why did one need to mimic another? The German naturalist Fritz Müller put forward the first explanation for this phenomenon: If two species were confused with one another by a common predator, individuals in both would be more likely to survive.[22][23] This type of mimicry is unique in several respects. Firstly, both the mimic and the model benefit from the interaction, which could thus be classified as mutualism in this respect. The signal receiver is also advantaged by this system, despite being deceived regarding species identity, as it avoids potentially harmful encounters. The usually clear identity of mimic and model are also blurred. In cases where one species is scarce and another abundant, the rare species can be said to be the mimic. When both are present in similar numbers however it is more realistic to speak of each as comimics than of a distinct 'mimic' and 'model' species, as their warning signals tend to converge toward something intermediate between the two.[24] Another theoretical problem comes up when one considers that the two species may exist on a continuum from the harmless to the highly noxious, raising the question of where Batesian mimicry ends and Müllerian convergence begins.[25][26]
Examples:
- Lepidoptera
- The Monarch Butterfly (Danaus plexippus) is a member of a Müllerian complex with the Viceroy butterfly (Limenitis archippus) in shared coloration patterns and display behavior. The Viceroy has subspecies with somewhat different coloration, each one very closely matching the local Danaus species. E.g., in Florida, the pairing is of the Viceroy and the Queen Butterfly, and in Mexico, the Viceroy resembles the Soldier Butterfly. Therefore, the Viceroy is a single species involved in three different Müllerian pairs.[27] This example was long believed to be a case of Batesian mimicry, with the Viceroy being the mimic and the Monarch the model, but it was more recently determined that the Viceroy is actually the more unpalatable species, though there is considerable individual variation.[28] While L. archippus is really bad-tasting, Danaus species tend to be toxic rather than just repugnant, due to their different food plants.
- Unpalatable Euploea species look very similar. See also under Batesian mimicry above.
- The genus Morpho is palatable but are very strong fliers; birds – even species which are specialized for catching butterflies on the wing – find it very hard to catch them. The conspicuous blue coloration shared by most Morpho species seems to be a case of Müllerian mimicry.[18]
- The "orange complex" of species, including the heliconiines Agraulis vanillae, Dryadula phaetusa, and Dryas iulia which all taste bad.[18]
- Many different tiger moths make ultrasonic clicking calls to warn bats that they are unpalatable. Presumably a bat may learn to avoid any signalling moths, which would make this an example of Müllerian mimicry.[19]
- Various bees and numerous vespid and sphecoid wasps: These animals are examples of Müllerian mimics because they have the aposematic yellow and black stripes (sometimes black and red, or black and white). Females of most of these species are potentially harmful to predators, fulfilling the second requirement of Müllerian mimicry. However, in essentially all such species, the males are harmless, and can thus be considered automimics of their conspecific females (see below). There are also many genera in these groups where the females are not capable of stinging, and yet still possess aposematic coloration (e.g., the wasp genus Cerceris), so they are considered Batesian mimics.
Emsleyan/Mertensian

Emsleyan[8] or Mertensian mimicry describes unusual cases where deadly prey mimic a less dangerous species. It was first proposed by Emsley[29] as a possible answer for the problem of Coral Snake mimicry in the New World. It was elaborated on by the German biologist Wolfgang Wickler in a chapter of Mimicry in Plants and Animals,[3] who named it after the German herpetologist Robert Mertens.[30] Sheppard points out that Hecht and Marien put forward a similar hypothesis ten years earlier.[31]
This scenario is a little more difficult to understand, as in other types of mimicry it is usually the most harmful species that is the model. But if a predator dies, it cannot learn to recognize a warning signal, e.g. bright colors in a certain pattern. In other words, there is no advantage in being aposematic for an organism that is likely to kill any predator it succeeds in poisoning; such an animal would rather profit from being camouflaged, to avoid attacks altogether. If, however, there is some other species that is harmful but not deadly as well as aposematic, the predator may learn to recognize its particular warning colors and avoid such animals. A deadly species will then profit by mimicking the less dangerous aposematic organism, if this results in less attacks than camouflage would.
The exception here, ignoring any chance of animals learning by watching a conspecific die (see Jouventin et al. for a discussion of observational learning and mimicry),[32] is the possibility of not having to learn that it is harmful in the first place: instinctive genetic programming to be wary of certain signals. In this case, other organisms could benefit from this programming, and Batesian or Müllerian mimics of it could potentially evolve. In fact, it has been shown that some species do have an innate recognition of certain aposematic warnings. Hand-reared Turquoise-browed Motmots (Eumomota superciliosa), avian predators, instinctively avoid snakes with red and yellow rings.[33] Other colors with the same pattern, and even red and yellow stripes with the same width as rings, were tolerated. However, models with red and yellow rings were feared, with the birds flying away and giving alarm calls in some cases. This provides one alternative explanation to Mertensian mimicry. See Greene and McDiarmid for a review of the subject.[34]
Examples:
- Some Milk Snake (Lampropeltis triangulum) subspecies (harmless), the moderately toxic False Coral Snakes (genus Erythrolamprus), and the deadly Coral Snakes all have a red background color with black and white/yellow stripes. In this system, both the milk snakes and the deadly coral snakes are mimics, whereas the false coral snakes are the model.
Wasmannian
Wasmannian mimicry refers to cases where the mimic resembles a model along with which it lives (inquiline) in a nest or colony. Most of the models here are social insects such as ants, termites, bees and wasps.[35]
Mimetic weeds
Vavilovian mimicry describes weeds which come to share characteristics with a domesticated plant through artificial selection.[8] It is named after Russian botanist and geneticist Nikolai Vavilov.[36] Selection against the weed may occur either by manually killing the weed, or separating its seeds from those of the crop. The latter process, known as winnowing, can be done manually or by a machine.
Vavilovian mimicry presents an illustration of unintentional (or rather 'anti-intentional') selection by man. While some cases of artificial selection go in the direction desired, such as selective breeding, this case presents the opposite characteristics. Weeders do not want to select weeds that look increasingly like the cultivated plant, yet there is no other option. A similar problem in agriculture is pesticide. Vavilovian mimics may eventually be domesticated themselves, and Vavilov called these weeds-come-crops secondary crops.
It can be classified as defensive mimicry in that the weed mimics a protected species. This bears strong similarity to Batesian mimicry in that the weed does not share the properties that give the model its protection, and both the model and the dupe (in this case people) are harmed by its presence. There are some key differences, though; in Batesian mimicry the model and signal receiver are enemies (the predator would eat the protected species if could), whereas here the crop and its human growers are in a mutualistic relationship: the crop benefits from being dispersed and protected by people, despite being eaten by them. In fact, the crop's only 'protection' relevant here is its usefulness to humans. Secondly, the weed is not eaten, but simply destroyed. The only motivation for killing the weed is its effect on crop yields. Finally, this type of mimicry does not occur in ecosystems unaltered by humans.
One case is Echinochloa oryzoides, a species of grass which is found as a weed in rice (Oryza sativa) fields. The plant looks similar to rice and its seeds are often mixed in rice and difficult to separate. This close similarity was enhanced by the weeding process which is a selective force that increases the similarity of the weed in each subsequent generation.[37]
Protective egg decoys
Unlike the above forms of mimicry, Gilbertian mimicry involves only two species. The potential host/prey drives away its parasite/predator by mimicking it, the reverse of host-parasite aggressive mimicry. It was coined by Pasteur as a term for such rare mimicry systems,[8] and is named after the American ecologist Lawrence E. Gilbert.[38]
This form of protective mimicry occurs in the genus Passiflora. The leaves of this plant contain toxins which deter herbivorous animals, however some Heliconius butterfly larvae have evolved enzymes which break down these toxins, allowing them to specialize on this genus. This has created further selection pressure on the host plants, which have evolved stipules that mimic mature Heliconius eggs near the point of hatching. These butterflies tend to avoid laying eggs near each existing ones, which helps avoid exploitative intraspecific competition between caterpillars—those that lay on vacant leaves provide their offspring with a greater chance of survival. Additionally, most Heliconius larvae are cannibalistic, meaning those leaves with older eggs will hatch first and eat the new arrivals. Thus, it seems such plants have evolved egg dummies due to these grazing herbivore enemies. The decoy eggs are also nectaries though, attracting predators of the caterpillars such as ants and wasps. The extent of their mimetic function is therefore slightly more difficult to assess.[10]
The use of eggs is not essential to this system, only the species composition and protective function. Many other forms of mimicry also involve eggs, such as cuckoo eggs mimicking those of their host (the reverse of this situation), or plants seeds (often those with an elaiosome) being dispersed by ants, who treat them as they would their own eggs.
Protective mimicry within a species

Browerian mimicry[8], named after Lincoln P. Brower and Jane Van Zandt Brower,[39][40] is a form of automimicry; where the model belongs to the same species as the mimic. This is the analogue of Batesian mimicry within a single species, and occurs when there is a palatability spectrum within a population. One example is Monarch Butterflies (Danaus plexippus), which feed on milkweed species of varying toxicity. This species stores toxins from its host plant, which are maintained even in the adult (imago) form. As the levels of toxin will vary depending on diet during the larval stage, some individuals will be more toxic than others. The less palatable organisms will therefore be mimics of the more dangerous individuals, with their likeness already perfected. This need not be the case however; in sexually dimorphic species one sex may be more of a threat than the other, which could mimic the protected sex. Evidence for this possibility is provided by the behavior of a monkey from Gabon, which regularly ate male moths of the genus Anaphe, but promptly stopped after it tasted a noxious female.[41]
Aggressive
Aggressive mimicry describes predators (or parasites) which share the same characteristics as a harmless species, allowing them to avoid detection by their prey (or host). The mimic may resemble the prey or host itself, or another organism which is either neutral or beneficial to the signal receiver. In this class of mimicry the model may be affected negatively, positively or not at all. Just as parasites can be treated as a form of predator,[42] host-parasite mimicry is treated here as a subclass of aggressive mimicry.
The mimic may have a particular significance for duped prey. One such case is spiders, amongst which aggressive mimicry is quite common in both luring prey and stealthily approaching predators.[43] One case is the Golden Orb Weaver (Nephila clavipes), which spins a conspicuous golden colored web in well-lit areas. Experiments show that bees are able to associate the webs with danger when the yellow pigment is not present, as occurs in less well-lit areas where the web is much harder to see. Other colors were also learned and avoided, but bees seemed least able to effectively associate yellow pigmented webs with danger. Yellow is the color of many nectar bearing flowers, however, so perhaps avoiding yellow is not worth while. Another form of mimicry is based not on color but pattern. Species such as Argiope argentata employ prominent patterns in the middle of their webs, such as zigzags. These may reflect ultraviolet light, and mimic the pattern seen in many flowers known as nectar guides. Spiders change their web day to day, which can be explained by bee's ability to remember web patterns. Bees are able to associate a certain pattern with a spatial location, meaning the spider must spin a new pattern regularly or suffer diminishing prey capture.[44]
Another case is where males are lured towards what would seem to be a sexually receptive female; the model in this situation being the same species as the dupe. Beginning in the 1960s, James E. Lloyd's investigation of female fireflies of the genus Photuris revealed they emit the same light signals that females of the genus Photinus use as a mating signal.[45] Further research showed male fireflies from several different genera are attracted to these "femmes fatales", and are subsequently captured and eaten. Female signals are based on that received from the male, each female having a repertoire of signals matching the delay and duration of the female of the corresponding species. This mimicry may have evolved from non-mating signals that have become modified for predation.[46]

The listrosceline katydid Chlorobalius leucoviridis of inland Australia is capable of attracting male cicadas of the Tribe Cicadettini by imitating the species-specific reply clicks of sexually receptive female cicadas. This example of acoustic aggressive mimicry is similar to the Photuris firefly case in that the predator's mimicry is remarkably versatile – playback experiments show that C. leucoviridis is able to attract males of many cicada species, including Cicadettine cicadas from other continents, even though cicada mating signals are species-specific.[47]
Some carnivorous plants may also be able to increase their rate of capture through mimicry.[48]

Luring is not a necessary condition however, as the predator will still have a significant advantage by simply not being identified as such. They may resemble a mutualistic symbiont or a species of little relevance to the prey.
A case of the former situation is a species of cleaner fish and its mimic, though in this example the model is greatly disadvantaged by the presence of the mimic. Cleaner fish are the allies of many other species, which allow them to eat their parasites and dead skin. Some allow the cleaner to venture inside their body to hunt these parasites. However, one species of cleaner, the Bluestreak cleaner wrasse (Labroides dimidiatus), is the unknowing model of a mimetic species, the Sabre-toothed blenny (Aspidontus taeniatus). This wrasse, shown to the left cleaning a grouper of the genus Epinephelus, resides in coral reefs in the Indian and the Pacific Oceans, and is recognized by other fishes who then allow it to clean them. Its imposter, a species of blenny, lives in the Indian Ocean and not only looks like it in terms of size and coloration, but even mimics the cleaner's 'dance'. Having fooled its prey into letting its guard down, it then bites it, tearing off a piece of its fin before fleeing the scene. Fish grazed upon in this fashion soon learn to distinguish mimic from model, but because the similarity is close between the two they become much more cautious of the model as well, such that both are affected. Due to victim's ability to discriminate between foe and helper, the blennies have evolved close similarity, right down to the regional level.[49]
Another interesting example that does not involve any luring is the Zone-tailed Hawk, which resembles the Turkey Vulture. It flies amongst the vultures, suddenly breaking from the formation and ambushing its prey.[50] Here the hawk's presence is of no evident significance to the vultures, affecting them neither negatively or positively.
Parasites
Parasites can also be aggressive mimics, though the situation is somewhat different from those outlined above.
Some of the predators described have a feature that draws prey, and parasites can also mimic their host's natural prey, but are eaten themselves, a pathway into their host. Leucochloridium, a genus of flatworm, matures in the digestive system of songbirds, their eggs then passing out of the bird via the feces. They are then taken up by Succinea, a terrestrial snail. The eggs develop in this intermediate host, and then must find of a suitable bird to mature in. As the host birds do not eat snails, so the sporocyst has another strategy to reach its host's intestine. They are brightly colored and move in a pulsating fashion. A sporocyst-sac pulsates in the snail's eye stalks,[51][52] coming to resemble an irresistible meal for a songbird. In this way, it can bridge the gap between hosts, allowing it to complete its life cycle.[3] A nematode (Myrmeconema neotropicum) changes the colour of the abdomen of workers of the canopy ant Cephalotes atratus to make it appear like the ripe fruits of Hyeronima alchorneoides. It also changes the behaviour of the ant so that the gaster (rear part) is held raised. This presumably increases the chances of the ant being eaten by birds. The droppings of birds are collected by other ants and fed to their brood, thereby helping to spread the nematode.[53]
In an unusual case, planidium larvae of some beetles of the genus Meloe will form a group and produce a pheromone that mimics the sex attractant of its host bee species; when the male bee arrives and attempts to mate with the mass of larvae, they climb onto his abdomen, and from there transfer to a female bee, and from there to the bee nest to parasitize the bee larvae.[54]
Host-parasite mimicry is a two species system where a parasite mimics its own host. Cuckoos are a canonical example of brood parasitism, a form of kleptoparasitism where the mother has its offspring raised by another unwitting organism, cutting down the biological mother's parental investment in the process. The ability to lay eggs which mimic the host eggs is the key adaptation. The adaptation to different hosts is inherited through the female line in so-called gentes. Cases of intraspecific brood parasitism, where a female lays in conspecific's nest, as illustrated by the Goldeneye duck (Bucephala clangula),[55] do not represent a case of mimicry.
Reproductive
Reproductive mimicry occurs when the actions of the dupe directly aid in the mimic's reproduction. This is common in plants, which may have deceptive flowers that do not provide the reward they would seem to. Other forms of mimicry have a reproductive component, such as Vavilovian mimicry involving seeds, and brood parasitism, which also involves aggressive mimicry.
Mimicry of flowers
Bakerian mimicry, named after Herbert G. Baker,[56] is a form of automimicry where female flowers mimic male flowers of their own species, cheating pollinators out of a reward. This reproductive mimicry may not be readily apparent as members of the same species may still exhibit some degree of sexual dimorphism. It is common in many species of Caricaceae.[57]
Like Bakerian mimicry, Dodsonian mimicry is a form of reproductive floral mimicry, but the model belongs to a different species than the mimic. The name refers to Calaway H. Dodson.[58] By providing similar sensory signals as the model flower, it can lure its pollinators. Like Bakerian mimics, no nectar is provided. Epidendrum ibaguense of the family Orchidaceae resembles flowers of Lantana camara and Asclepias curassavica, and is pollinated by Monarch Butterflies and perhaps hummingbirds.[59] Similar cases are seen in some other species of the same family. The mimetic species may still have pollinators of its own though, for example a lamellicorn beetle which usually pollinates correspondingly colored Cistus flowers is also known to aid in pollination of Ophrys species that are normally pollinated by bees.[60]
Pseudocopulation

Pseudocopulation occurs when a flower mimics a female of a certain insect species, the males of which try to copulate with it. This is much like the aggressive mimicry in fireflies described above, but with a much more benign outcome for the pollinator. This form of mimicry has been called Pouyannian mimicry,[8] after Pouyanne, who first described the phenomenon.[61][62] It is most common in orchids which mimic females of the order Hymenoptera (generally bees and wasps), and may account for around 60% of pollinations.[63] Depending on the morphology of the flower, a pollen sac called a pollinia is attached to the head or abdomen of the male. This is then transferred to the stigma of the next flower the male tries to inseminate, resulting in pollination. Visual mimicry is the most obvious sign of this deception for humans, but the visual aspect may be minor or non-existent. It is the senses of touch and olfaction that are most important.[63]
Inter-sexual mimicry
Inter-sexual mimicry occurs when individuals of one sex in a species mimic members of the opposite sex. An example is the three male forms of the marine isopod, Paracerceis sculpta. Alpha males are the largest and guard a harem of females. Beta males mimic females and manage to enter the harem of females without being detected by the alpha males allowing them to mate. Gamma males are the smallest males and mimic juveniles. This also allows them to mate with the females without the alpha males detecting them.[64] Some male Australian Giant Cuttlefish also mimic females, allowing them to mate undetected by other males.
Automimicry
Automimicry or intraspecific mimicry occurs within a single species, one case being where one part of an organism's body resembles another part. Examples include snakes in which the tail resembles the head and show behavior such as moving backwards to confuse predators and insects and fishes with eyespots on their hind ends to resemble the head. The term is also used when the mimic imitates other morphs within the same species. When males mimic females or vice versa this may be referred to as sexual mimicry.
Examples:
- Many insects have filamentous "tails" at the ends of their wings which are combined with patterns of markings on the wings themselves to create a "false head" which misdirects predators (e.g., hairstreak butterflies).
- Several pygmy owls bear "false eyes" on the back of their head to fool predators into believing the owl is alert to their presence.
- The yellow throated males of the Common Side-blotched Lizard use a 'sneaking' strategy in mating. They look and behave like unreceptive females. This strategy is effective against 'usurper' males with orange throats, but ineffective against blue throated 'guarder' males, which will chase them away.[65]
- Female hyenas have pseudo-penises which make them look like males.[66]
Other
Some forms of mimicry do not fit easily within the classification given above.
Owl butterflies (genus Caligo) bear eye-spots on the underside of their wings; if turned upside-down, their undersides resemble the face of an owl (such as the Short-eared Owl or the Tropical Screech Owl) for which in turn the butterfly predators – small lizards and birds – would be fooled.[67] Thus it has been supposed that the eye-spots are a form of Batesian mimicry. However, the pose in which the butterfly resembles an owl's head is not normally adopted in life. Research suggests that eye-spots are not a form of mimicry and do not deter predators because they look like eyes. Rather the conspicuous contrast in the patterns on the wings deter predators.[68]
Another case is floral mimicry induced by the discomycete fungus Monilinia vaccinii-corymbosi.[69] In this unusual case, a fungal plant pathogen infects leaves of blueberries, causing them to secrete sugary substances including glucose and fructose, in effect mimicking the nectar of flowers. To the naked eye the leaves do not look like flowers, yet strangely they still attract pollinating insects like bees. As it turns out, the sweet secretions are not the only cues—the leaves also reflect ultraviolet, which is normally absorbed by the plant's leaves. Ultraviolet light is also employed by the host's flowers as a signal to insects, which have visual systems quite capable of picking up this low wavelength (300–400 nm) radiation. The fungus is then transferred to the ovaries of the flower where it produces mummified, inedible berries, which overwinter before infecting new plants. This case is unusual in that the fungus benefits from the deception, but it is the leaves which act as mimics, being harmed in the process. It bears similarity to host-parasite mimicry, but the host does not receive the signal. It also has a little in common with automimicry, but the plant does not benefit from the mimicry, and the action of the pathogen is required to produce it.
Evolution

It is widely accepted that mimicry evolves as a positive adaptation; that is, the mimic gains fitness via convergent evolution which results in resemblance to another species. The lepidopterist (and sometime author) Vladimir Nabokov argued that much of insect mimicry, including the Viceroy/Monarch mimicry, resulted from the fact that coloration patterns in both species simply had a common structural basis, and thus the tendency for convergence by chance was high.[70] However, this very example provides evidence to the contrary. The viceroy's color pattern is completely unlike any of the species to which it is closely related, and the viceroy itself has three color forms, each adapted to resemble a different species of Danaus.[27] Also, many cases of mimicry (especially in large Batesian/Mũllerian complexes) involve insects from multiple orders that share virtually no structural similarities whatsoever; beetles, true bugs, moths, wasps, bees, and flies may all belong to a single mimetic complex, despite their biological differences.[3]
The most widely accepted model used to explain the evolution of mimicry in butterflies is the two-step hypothesis. In this model the first step involves mutation in modifier genes that regulate a complex cluster of linked genes associated with large changes in morphology. The second step consists of selections on genes with smaller phenotypic effects and this leading to increasing closeness of resemblance. This model is supported by empirical evidence that suggests that there are only a few single point mutations that cause large phenotypic effects while there are numerous others that produce smaller effects. Some regulatory elements are now known to be involved in a supergene that is involved in the development of butterfly color patterns. Computational simulations of population genetics have also supported this idea.[71]
See also
|
|
Similar terms
- Mimetic is an adjective used to describe cases of mimicry, but is also used in mathematics (see mimetic). This should not be confused with memetics, the scientific study of memes.
- Mimesis also refers to imitation, especially relating to the arts.
Further reading
- Vane-Wright RI. 1976. A unified classification of mimetic resemblances. Biol. J. Linn. Soc. 8:25–56
- Cott, H.B. (1940) Adaptive Coloration in Animals. Methuen and Co, Ltd., London ISBN 0-416-30050-2
- Wickler, W. (1968) Mimicry in Plants and Animals (Translated from the German) McGraw-Hill, New York. ISBN 0-07-070100-8
- Edmunds, M. 1974. Defence in Animals: A Survey of Anti-Predator Defences. Harlow, Essex and NY: Longman 357 p. ISBN 0-582-44132-3
- Owen, D. (1980) Camouflage and Mimicry. Oxford University Press ISBN 0-19-217683-8
- Pasteur, Georges (1982). "A classificatory review of mimicry systems". Annual Review of Ecology and Systematics 13: 169–199.
- Brower, L. (ed., 1988). Mimicry and the Evolutionary Process. Chicago: The University of Chicago Press. ISBN 0-226-07608-3 (a supplement of volume 131 of the journal American Naturalist dedicated to E. B. Ford.)
- Ruxton, G. D.; Speed, M. P.; Sherratt, T. N. (2004). Avoiding Attack. The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford: Oxford University Press. ISBN 0-19-852860-4
- Evans, M. A. (1965) Mimicry and the Darwinian Heritage Journal of the History of Ideas 26 (2): 211–220.
- Wiens, D. (1978) Mimicry in Plants. Evolutionary Biology. 11:365–403.
- Dafni, A. (1984) Mimicry and Deception in Pollination Annual Review of Ecology and Systematics 15: 259–278.
- An introductory book for a younger audience: Hoff, M. K. (2003) Mimicry and Camouflage. Creative Education. Mankato, Minn. Great Britain. ISBN 1-58341-237-9
References
- ↑ Less commonly known as mimetism.
- ↑ King R.C. Stansfield W.D. and Mulligan P.K. 2006. A dictionary of genetics, 7th ed. Oxford. p278
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Wickler W. 1968. Mimicry in plants and animals. McGraw-Hill, New York
- ↑ This 'group' is often a species, but can also be a subgroup such as one particular sex or morph
- ↑ In its broadest definition mimicry can include non-living models.
- ↑ 6.0 6.1 Wickler, W. (1965). "Mimicry and the evolution of animal communication". Nature 208: 519–21. doi:10.1038/208519a0.
- ↑ "A moth in spider's clothing <<Neurophilosophy". 2006-12-22. http://neurophilosophy.wordpress.com/2006/12/22/the-moth-in-spiders-clothing/. Retrieved 2008-06-07. (includes video)
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Pasteur G. 1982. A classificatory review of mimicry systems. Annual Review of Ecology and Systematics 13, 169–199.
- ↑ 9.0 9.1 Ruxton G.D. Sherratt T.N. and Speed M.P. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. Oxford.
- ↑ 10.0 10.1 Campbell, N. A. (1996) Biology (4th edition), Chapter 50. Benjamin Cummings, New York ISBN 0-8053-1957-3
- ↑ Boyden T.C. 1980. Floral mimicry by Epidendrum ibaguense (Orchidaceae) in Panama. Evolution 34:135–136.
- ↑ Roy B.A. 1994. The effects of pathogen-induced pseudoflowers and buttercups on each other's insect visitation Ecology 75:352–358.
- ↑ Wickler, Wolfgang 1998. "Mimicry". Encyclopædia Britannica, 15th edition. Macropædia 24, 144–151. http://www.britannica.com/eb/article-11910
- ↑ Douglas Harper (2007-10-06). "Online Etymology Dictionary". http://www.etymonline.com/index.php?search=mimicry&searchmode=none.
- ↑ Davies, NB and JA Welbergen (2008). "Cuckoo–hawk mimicry? An experimental test". Proceedings of the Royal Society B: Biological Sciences 275 (1644): 1817–1822. doi:10.1098/rspb.2008.0331. PMID 18467298.
- ↑ Bates H. W. 1863. The naturalist on the river Amazons. Murray, London.
- ↑ Bates, H. W. (1961) Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Transactions of the Linnean Society. 23:495–566.
- ↑ 18.0 18.1 18.2 18.3 Pinheiro, Carlos E. G. (1996) Palatability and escaping ability in Neotropical butterflies: tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae). Biol. J. Linn. Soc. 59(4): 351–365. HTML abstract
- ↑ 19.0 19.1 Barber, J. R. and W. E. Conner. (2007) Acoustic mimicry in a predator–prey interaction. Proc. Nat. Acad. Sci. 104(22):9331–9334 [1]
- ↑ Mimic Octopus, Thaumoctopus mimicus at MarineBio.org
- ↑ Meyer A (2006) Repeating Patterns of Mimicry. PLoS Biol 4(10): e341 doi:10.1371/journal.pbio.0040341
- ↑ Müller, Fritz (1878) Ueber die Vortheile der Mimicry bei Schmetterlingen. Zoologischer Anzeiger 1: 54–55.
- ↑ Müller, F. (1879) Ituna and Thyridia; a remarkable case of mimicry in butterflies. (R. Meldola translation) Proclamations of the Entomological Society of London 1879:20–29.
- ↑ Flannery, T. F. (2007) "Community ecology: Mimicry complexes". Encyclopædia Britannica Online. http://www.britannica.com/eb/article-9117280/community-ecology
- ↑ Huheey, James E. (1976) Studies in warning coloration and mimicry VII. Evolutionary consequences of a Batesian–Müllerian spectrum: A model for Müllerian mimicry. Evolution 30:86–93.
- ↑ Benson, W. W. (1977) On the Supposed Spectrum Between Batesian and Mullerian Mimicry. Evolution. 31:454–455.
- ↑ 27.0 27.1 Ritland, D.B. 1995. Comparative unpalatability of mimetic viceroy butterflies (Limenitis archippus) from four south-eastern United States populations. Oecologia 103: 327–336
- ↑ Ritland, D.; L. P. Brower (1991). "The viceroy butterfly is not a Batesian mimic". Nature 350: 497–498. doi:10.1038/350497a0. http://www.nature.com/nature/journal/v350/n6318/abs/350497a0.html. Retrieved 2008-02-23. "Viceroys are as unpalatable as monarchs, and significantly more unpalatable than queens from representative Florida populations.".
- ↑ Emsley, M. G. (1966) The mimetic significance of Erythrolamprus aesculapii ocellatus Peters from Tobago. Evolution 20:663–64.
- ↑ Mertens R. 1956. Das Problem der Mimikry bei Korallenschlangen. Zool. Jahrb. Syst. 84:541–76.
- ↑ Hecht M.K. and Marien D. 1956. The coral snake mimic problem: a reinterpretation. Journal of Morphology. 98:335–365 see Sheppard P.M. 1969. Review of Mimicry in plants and animals by Wolfgang Wickler Journal of Animal Ecology 38: 243.
- ↑ Jouventin, P.; G. Pasteur; J. P. Cambefort (1977) Observational Learning of Baboons and Avoidance of Mimics: Exploratory Tests Evolution 31:214–218.
- ↑ Smith, S. M. (1975) Innate Recognition of Coral Snake Pattern by a Possible Avian Predator. Science. 187:759–760.
- ↑ Greene, H. W., McDiarmid, R. W. (1981) Coral snake mimicry: Does it occur? Science 213:1207–12.
- ↑ Wasmann, E. 1894. Kritisches Verzeichniss der myrmecophilin und termitophilen Arthropoden. Felix Dames, Berlin xi + 231 pp.
- ↑ Vavilov, N. I. (1951) The origin, variation, immunity and breeding of cultivated plants. (Translation by K. S. Chester) Chronica Botanica 13:1–366.
- ↑ Barrett, S. (1983) Mimicry in Plants Scientific American. 257: 76–83.
- ↑ Gilbert, L. E. (1975) Ecological consequences of a coevolved mutualism between butterflies and plants. In L. E. Gilbert, P. H. Raven (eds.) Coevolution of Animal and Plants pp. 210–40. Austin and London: University of Texas Press
- ↑ Brower, L. P. (1970) Plant poisons in a terrestrial food chain and implications for mimicry theory. In K. L. Chambers (ed) Biochemical Coevolution Corvallis, OR: Oregon State Univ. pp. 69–82.
- ↑ Brower, L. P., Brower, J. V. Z., Corvino, J. M. (1967) Plant poisons in a terrestrial food chain. Proceedings of the National Academy of Sciences USA 57:893–98.
- ↑ Bigot, L., Jouventin, P. (1974) Quelques expériences de comestibilité de Lépidoptères gabonais faites avec le mandrill, le cercocèbe à joues grises et legarde-boeufs. Terre Vie 28:521–43.
- ↑ Begon, M., Townsend, C., Harper, J. (1996) Ecology: Individuals, populations and communities (Third edition) Blackwell Science, London
- ↑ Jackson, R. R. (1995) Eight-legged tricksters: Spiders that specialize at catching other spiders. BioScience 42:590–98.
- ↑ Craig, C. L. (1995) Webs of Deceit. Natural History 104 (3): 32–35.
- ↑ Lloyd, J. E. (1965) Aggressive Mimicry in Photuris: Firefly Femmes Fatales Science 149:653–654.
- ↑ Lloyd, J. E. (1975) Aggressive Mimicry in Photuris Fireflies: Signal Repertoires by Femmes Fatales. Science. 187:452–453.
- ↑ Marshall, D. C. and K. B. R. Hill. 2009. Versatile aggressive mimicry of cicadas by an Australian predatory katydid. PLoS One. 4:1 e4185
- ↑ Moran, Jonathan A. (1996) Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology 84:515–525.
- ↑ Wickler, W. (1966) Mimicry in Tropical Fishes. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 251:473–474.
- ↑ Willis, E. O. (1963) Is the Zone-Tailed Hawk a Mimic of the Turkey Vulture? The Condor 65:313–317.
- ↑ See here for a photo.
- ↑ Moore J. 2002. Parasites and the behavior of animals. Oxford University Press, Oxford.
- ↑ Yanoviak S.P. M. Kaspari, R. Dudley and G. Poinar Jr. 2008. Parasite-induced fruit mimicry in a tropical canopy ant. The American Naturalist 171: 536–544. PDF
- ↑ Leslie Saul-Gershenz 2007. Bee nest parasites (Meloe franciscanus) use sexual deception to obtain transport to host bee (Habropoda pallida) nest. ESA Annual Meeting, 2007 Abstract
- ↑ Andersson, M. and Eriksson, M.O.G. 1982 Nest parasitism in Goldeneyes Bucephala clangula: some evolutionary aspects. American Naturalist 120, 1–16.
- ↑ Baker H. G. 1976. "Mistake" pollination as a reproductive system, with special reference to the Caricaceae. Pp 161–169 in J. Burley and B.T. Styles, eds. Variation, breeding, and conservation of tropical trees. Academic Press, London, U.K.
- ↑ Bawa, K. S. (1980) Mimicry of male by female flowers and intrasexual competition for pollinators in Jacaratia dolichaula (D. Smith) Woodson (Caricaceae). Evolution 34:467–74.
- ↑ Dodson, C. H., Frymire, G. P. (1961) Natural pollination of orchids. Missouri Botanical Garden Bulletin 49:133–39.
- ↑ Boyden, T. C. (1980) Floral mimicry by Epidendrurn ibaguense (Orchidaceae) in Panama. Evolution 34:135–36.
- ↑ Kullenberg, B. (1961) Studies in Ophrys pollination. Zool. Bidr. Uppsala 34:1–340.
- ↑ Correvon H., Pouyanne A. (1916) Uncurieux cas de mimetisme chez les Ophrydees. J. Soc. Nat. Hortic. Fr. 17:29–31, 41–42, 84.
- ↑ Pouyanne, M. (1917) La fécondation des Ophrys par les insectes. Bull. Soc. Hist. Nat. Afr. Nord 8:1–2.
- ↑ 63.0 63.1 van der Pijl, L., Dodson, C. H. (1966) Orchid Flowers; Their Pollination and Evolution. Coral Gables, FL: Univ. Miami Press
- ↑ Shuster, Stephen (May 1987). "Alternative Reproductive Behaviors: Three Discrete Male Morphs in Paracerceis sculpta, an Intertidal Isopod from the Northern Gulf of California". Journal of Crustacean Biology 7 (2): 318–327. doi:10.2307/1548612. http://www.jstor.org/stable/1548612. Retrieved 20 May 2009 Free Version.
- ↑ Sinervo B.; Miles D.B.; Frankino W.A.; Klukowski M.; DeNardo D.F. (2000) Testosterone, Endurance, and Darwinian Fitness: Natural and Sexual Selection on the Physiological Bases of Alternative Male Behaviors in Side-Blotched Lizards. Hormones and Behavior. 38:222–233.
- ↑ Muller, M. N.; Wrangham, R. (2002) Sexual Mimicry in Hyenas The Quarterly Review of Biology 77:3–16.
- ↑ See here for a photo
- ↑ Martin Stevens, Chloe J. Hardman, and Claire L. Stubbins Conspicuousness, not eye mimicry, makes eyespots effective antipredator signals; Behavioral Ecology; Volume 19, Number 3, pp. 525–531 doi:10.1093/beheco/arm162
- ↑ Batra L. R.; Batra, S. (1985) Floral Mimicry Induced by Mummy-Berry Fungus Exploits Host's Pollinators as Vectors Science 228:1011–1013.
- ↑ Alexander, Victoria N. Nabokov and Insect mimicry. Nabokov Studies
- ↑ Holmgren N.M.A and M. Enquist 1999. Dynamics of mimicry evolution. Biological Journal of the Linnean Society. 66:145–158. PDF
External links
- Warning Colour and Mimicry Lecture outline from University College London
- Camouflage and Mimicry in Fossils
- Chemical Mimicry in Pollination
- Learn about Butterflies Simple account.
|
|||||
|
||||||||||||||||
|
||||||||
