Vasopressin
| edit |
| Arginine vasopressin (neurophysin II, antidiuretic hormone, diabetes insipidus, neurohypophyseal) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
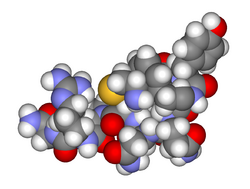 Space-filling model of arginine vasopressin |
|||||||||||||
|
|||||||||||||
| Identifiers | |||||||||||||
| Symbols | AVP; ADH; AVP-NPII; VP | ||||||||||||
| External IDs | OMIM: 192340 MGI: 88121 HomoloGene: 417 GeneCards: AVP Gene | ||||||||||||
|
|||||||||||||
| RNA expression pattern | |||||||||||||
 |
|||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 551 | 11998 | |||||||||||
| Ensembl | ENSG00000101200 | ENSMUSG00000037727 | |||||||||||
| UniProt | P01185 | Q3UUQ5 | |||||||||||
| RefSeq (mRNA) | NM_000490 | NM_009732 | |||||||||||
| RefSeq (protein) | NP_000481 | NP_033862 | |||||||||||
| Location (UCSC) | Chr 20: 3.01 - 3.01 Mb |
Chr 2: 130.27 - 130.27 Mb |
|||||||||||
| PubMed search | [1] | [2] | |||||||||||
Arginine vasopressin (AVP), also known as vasopressin, argipressin or antidiuretic hormone (ADH), is a hormone found in most mammals, including humans.[1] Vasopressin is a peptide hormone that controls the reabsorption of molecules in the tubules of the kidneys by affecting the tissue's permeability. It also increases peripheral vascular resistance, which in turn increases arterial blood pressure. It plays a key role in homeostasis, and the regulation of water, glucose, and salts in the blood. It is derived from a preprohormone precursor that is synthesized in the hypothalamus and stored in vesicles at the posterior pituitary. Most of it is stored in the posterior pituitary to be released into the bloodstream; however, some AVP is also released directly into the brain.
Contents |
Physiology
Function
One of the most important roles of AVP is to regulate the body's retention of water; it is released when the body is dehydrated and causes the kidneys to conserve water, thus concentrating the urine, and reducing urine volume. In high concentrations, it also raises blood pressure by inducing moderate vasoconstriction. In addition, it has a variety of neurological effects on the brain, having been found, for example, to influence pair-bonding in voles. The high-density distributions of vasopressin receptor AVPr1a in prairie vole ventral forebrain regions have been shown to facilitate and coordinate reward circuits during partner preference formation, critical for pair bond formation [2]
A very similar substance, lysine vasopressin (LVP) or lypressin, has the same function in pigs and is often used in human therapy.
Kidney
Vasopressin has three effects by which it contributes to increased urine osmolality (increased concentration) and decreased water excretion. These are:
1) Increase in the permeability to water of the cells of distal tubule and collecting duct in the kidney and thus allows water reabsorption and excretion of concentrated urine, i.e. antidiuresis. This occurs through insertion of water channels (Aquaporin-2) into the apical membrane of the distal tubule and collecting duct epithelial cells. The aquaporins allow water to move out of the nephron, increasing the amount of water re-absorbed from the forming urine back into the bloodstream.
V2 receptors, G protein-coupled receptors on the basolateral plasma membrane of the epithelial cells couple to the heterotrimeric G-protein Gs, which activates adenylyl cyclases III and VI to convert ATP into cAMP, plus 2 inorganic phosphates. The rise in cAMP then triggers the insertion of aquaporin-2 water channels by exocytosis of intracellular vesicles, recycling endosomes. Vasopressin also increases the concentration of calcium in the collecting duct cells, by episodic release from intracellular stores. Vasopressin, acting through cAMP also increases transcription of the aquaporin-2 gene, thus increasing the total number of aquaporin-2 molecules in collecting duct cells.
Cyclic-AMP activates protein kinase A (PKA)by binding to its regulatory subunits and allowing them to detach from the catalytic subunits. Detachment exposes the catalytic site in the enzyme, allowing it to add phosphate groups to proteins (including the aquaporin-2 protein), which alters their functions.
2) Increase in the permeability of the inner medullary portion of the collecting duct to urea, allowing increased reabsorption of urea into the medullary interstitium, down the concentration gradient created from the removal of water in the connecting tubule, cortical collecting duct, and outer medullary collecting duct.
3) Stimulation of sodium and chloride reabsorption in the thick ascending limb of the loop of Henle by increasing the activity of the Na+-K+-2Cl--cotransporter. NaCl reabsorption drives the process of countercurrent multiplication, which furnishes the osmotic gradient for aquaporin mediated water reabsorption in the medullary collecting ducts.
Cardiovascular system
Vasopressin increases peripheral vascular resistance and thus increases arterial blood pressure. This effect appears small in healthy individuals; however it becomes an important compensatory mechanism for restoring blood pressure in hypovolemic shock such as that which occurs during hemorrhage.
Central nervous system (CNS)
Vasopressin released within the brain has many actions:
- It has been implicated in memory formation, including delayed reflexes, image, short- and long-term memory, though the mechanism remains unknown; these findings are controversial. However, the synthetic vasopressin analogue desmopressin has come to interest as a likely nootropic.
- Vasopressin is released into the brain in a circadian rhythm by neurons of the Supraoptic nucleus.
- Vasopressin released from centrally-projecting hypothalamic neurons is involved in aggression, blood pressure regulation and temperature regulation.
- Selective AVPr1a blockade in the ventral pallidum has been shown to prevent partner preference, suggesting that these receptors in this ventral forebrain region are crucial for pair bonding. [2]
In recent years, there has been particular interest in the role of vasopressin in social behavior. It is thought that vasopressin, released into the brain during sexual activity, initiates and sustains patterns of activity that support the pair-bond between the sexual partners; in particular, vasopressin seems to induce the male to become aggressive towards other males.[3]
Evidence for this comes from experimental studies in several species, which indicate that the precise distribution of vasopressin and vasopressin receptors in the brain is associated with species-typical patterns of social behavior. In particular, there are consistent differences between monogamous species and promiscuous species in the distribution of AVP receptors, and sometimes in the distribution of vasopressin-containing axons, even when closely-related species are compared.[3] Moreover, studies involving either injecting AVP agonists into the brain or blocking the actions of AVP support the hypothesis that vasopressin is involved in aggression towards other males. There is also evidence that differences in the AVP receptor gene between individual members of a species might be predictive of differences in social behavior. One study has suggested that genetic variation in male humans effects pair-bonding behavior. The brain of males uses vasopressin as a reward for forming lasting bonds with a mate, and men with one or two of the genetic alleles are more likely to experience marital discord. The partners of the men with two of the alleles affecting vasopressin reception state disappointing levels of satisfaction, affection, and cohesion.[4] Vasopressin receptors distributed along the reward circuit pathway, to be specific in the ventral pallidum, are activated when AVP is released during social interactions such as mating, in monogamous prairie voles. The activation of the reward circuitry reinforces this behavior, leading to conditioned partner preference, and thereby initiates the formation of a pair bond.[5]
Control
Vasopressin is secreted from the posterior pituitary gland in response to reductions in plasma volume, in response to increases in the plasma osmolality, and in response to cholecystokinin by the small intestine:
- Secretion in response to reduced plasma volume is activated by pressure receptors in the veins, atria, and carotids.
- Secretion in response to increases in plasma osmotic pressure is mediated by osmoreceptors in the hypothalamus.
- Secretion in response to increases in plasma Cholecystokinin is mediated by an unknown pathway.
The neurons that make AVP, in the hypothalamic supraoptic nuclei (SON) and paraventricular nuclei (PVN), are themselves osmoreceptors, but they also receive synaptic input from other osmoreceptors located in regions adjacent to the anterior wall of the third ventricle. These regions include the organum vasculosum of the lamina terminalis and the subfornical organ.
Many factors influence the secretion of vasopressin:
- Ethanol (alcohol) acts as an antagonist for AVP in the collecting ducts of the kidneys, which prevents aquaporins from binding to the collecting ducts, and prevents water reabsorption.
- Angiotensin II may stimulate the secretion of AVP.[6]
Secretion
The main stimulus for secretion of vasopressin is increased osmolality of plasma. Reduced volume of extracellular fluid also has this effect, but is a less sensitive mechanism.
The AVP that is measured in peripheral blood is almost all derived from secretion from the posterior pituitary gland (except in cases of AVP-secreting tumours). However there are two other sources of AVP with important local effects:
- Vasopressin is produced in the PVN and SON and travels down the axons through the infundibulum within neurosecretory granules that are found within Herring bodies, localized swellings of the axons and nerve terminals. These carry the peptide directly to the posterior pituitary gland, where it is stored until released into the blood.
- Vasopressin is also released into the brain by several different populations of smaller neurons (see below).
Receptors
Below is a table summarizing some of the actions of AVP at its three receptors, differently expressed in different tissues and exerting different actions:
| Type | Second messenger system | Locations | Actions |
| AVPR1A | phosphatidylinositol/calcium | liver, kidney, peripheral vasculature, brain | vasoconstriction, gluconeogenesis, platelet aggregation, and release of factor VIII and von Willebrand factor; social recognition,[7] circadian tau[8] |
| AVPR1B | phosphatidylinositol/calcium | pituitary gland, brain | adrenocorticotropic hormone secretion in response to stress;[9] social interpretation of olfactory cues[10] |
| AVPR2 | adenylate cyclase/cAMP | basolateral membrane of the cells lining the collecting ducts of the kidneys (especially the cortical and outer medullary collecting ducts) | insertion of aquaporin-2 (AQP2) channels (water channels). This allows water to be reabsorbed down an osmotic gradient, and so the urine is more concentrated. Release of von Willebrand factor and surface expression of P-selectin through exocytosis of Weibel-Palade bodies from endothelial cells[11][12] |
| VACM-1 | phosphatidylinositol/calcium | vascular endothelium and renal collecting tubules | Increases cytosolic calcium and acts as an inverse agonist of cAMP accumulation [11] |
Structure and relation to oxytocin

The vasopressins are peptides consisting of nine amino acids (nonapeptides). (NB: the value in the table above of 164 amino acids is that obtained before the hormone is activated by cleavage). The amino acid sequence of arginine vasopressin is Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly, with the cysteine residues forming a sulfur bridge. Lysine vasopressin has a lysine in place of the arginine.
The structure of oxytocin is very similar to that of the vasopressins: It is also a nonapeptide with a disulfide bridge and its amino acid sequence differs at only two positions (see table below). The two genes are located on the same chromosome separated by a relatively small distance of less than 15,000 bases in most species. The magnocellular neurons that make vasopressin are adjacent to magnocellular neurons that make oxytocin, and are similar in many respects. The similarity of the two peptides can cause some cross-reactions: oxytocin has a slight antidiuretic function, and high levels of AVP can cause uterine contractions.
Here is a table showing the superfamily of vasopressin and oxytocin neuropeptides:
Vertebrate Vasopressin Family Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2 Argipressin (AVP, ADH) Most mammals Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Lys-Gly-NH2 Lypressin (LVP) Pigs, hippos, warthogs, some marsupials Cys-Phe-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH2 Phenypressin Some marsupials Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Arg-Gly-NH2 Vasotocin† Non-mammals Vertebrate Oxytocin Family Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2 Oxytocin (OXT) Most mammals, ratfish Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Ile-Gly-NH2 Mesotocin Most marsupials, all birds, reptiles, amphibians, lungfishes, coelacanths Cys-Tyr-Ile-Gln-Ser-Cys-Pro-Ile-Gly-NH2 Seritocin Frogs Cys-Tyr-Ile-Ser-Asn-Cys-Pro-Ile-Gly-NH2 Isotocin Bony fishes Cys-Tyr-Ile-Ser-Asn-Cys-Pro-Gln-Gly-NH2 Glumitocin Skates Cys-Tyr-Ile-Asn/Gln-Asn-Cys-Pro-Leu/Val-Gly-NH2 Various tocins Sharks Invertebrate VP/OT Superfamily Cys-Leu-Ile-Thr-Asn-Cys-Pro-Arg-Gly-NH2 Diuretic Hormone Locust Cys-Phe-Val-Arg-Asn-Cys-Pro-Thr-Gly-NH2 Annetocin Earthworm Cys-Phe-Ile-Arg-Asn-Cys-Pro-Lys-Gly-NH2 Lys-Connopressin Geography & imperial cone snail, pond snail, sea hare, leech Cys-Ile-Ile-Arg-Asn-Cys-Pro-Arg-Gly-NH2 Arg-Connopressin Striped cone snail Cys-Tyr-Phe-Arg-Asn-Cys-Pro-Ile-Gly-NH2 Cephalotocin Octopus Cys-Phe-Trp-Thr-Ser-Cys-Pro-Ile-Gly-NH2 Octopressin Octopus †Vasotocin is the evolutionary progenitor of all the vertebrate neurohypophysial hormones.[13]
Role in disease
Decreased vasopressin release or decreased renal sensitivity to AVP leads to diabetes insipidus, a condition featuring hypernatremia (increased blood sodium concentration), polyuria (excess urine production), and polydipsia (thirst).
High levels of AVP secretion (syndrome of inappropriate antidiuretic hormone, SIADH) and resultant hyponatremia (low blood sodium levels) occurs in brain diseases and conditions of the lungs (Small cell lung carcinoma). In the perioperative period, the effects of surgical stress and some commonly used medications (e.g., opiates, syntocinon, anti-emetics) lead to a similar state of excess vasopressin secretion. This may cause mild hyponatremia for several days.
Hyponatremia can be treated pharmaceutically through the use of vasopressin receptor antagonists. These include the approved drug Vaprisol and the phase III drug lixivaptan.
Alcohol Consumption
Upon excessive alcohol consumption, the vasopressin production is reduced significantly. The inability to store water in the kidneys will prove fatal to those who participate in heavy consumption of alcohol, due to the dehydration caused by dilute urine and vomit.
Pharmacology
Vasopressin analogues
Vasopressin agonists are used therapeutically in various conditions, and its long-acting synthetic analogue desmopressin is used in conditions featuring low vasopressin secretion, as well as for control of bleeding (in some forms of von Willebrand disease) and in extreme cases of bedwetting by children. Terlipressin and related analogues are used as vasoconstrictors in certain conditions. Use of vasopressin analogues for esophageal varices commenced in 1970.[14]
Vasopressin infusion has been used as a second line of management in septic shock patients not responding to high dose of inotropes (e.g., dopamine or norepinephrine). It had been shown to be more effective than epinephrine in asystolic cardiac arrest.[15] While not all studies are in agreement, a 2006 study of out-of hospital cardiac arrests has added to the evidence for the superiority of AVP in this situation, but these studies relied on sub-group analysis and better designed prospective studies show no benefit in ACLS.[16][17]
Vasopressin receptor inhibition
A vasopressin receptor antagonist is an agent that interferes with action at the vasopressin receptors. They can be used in the treatment of hyponatremia.[18]
References
- ↑ Caldwell HK, Young WS III (2006). "Oxytocin and Vasopressin: Genetics and Behavioral Implications". In Lajtha A, Lim R. Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides (3rd ed.). Berlin: Springer. pp. 573–607. ISBN 0-387-30348-0. http://refworks.springer.com/mrw/fileadmin/pdf/Neurochemistry/0387303480C25.PDF.
- ↑ 2.0 2.1 Lim MM, Young LJ (2004). "Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole". Neuroscience 125 (1): 35–45. doi:10.1016/j.neuroscience.2003.12.008. PMID 15051143. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T0F-4BYRRFP-1&_user=1512538&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000053401&_version=1&_urlVersion=0&_userid=1512538&md5=7ffbccd80f369411ae19d3e0139a6297.
- ↑ 3.0 3.1 Young LJ (October 2009). "The neuroendocrinology of the social brain". Front Neuroendocrinol 30 (4): 425–8. doi:10.1016/j.yfrne.2009.06.002. PMID 19596026. http://linkinghub.elsevier.com/retrieve/pii/S0091-3022(09)00041-7.
- ↑ Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P (September 2008). "Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans". Proc. Natl. Acad. Sci. U.S.A. 105 (37): 14153–6. doi:10.1073/pnas.0803081105. PMID 18765804.
- ↑ LJ Pitkow, CA Sharer, X Ren, TR Insel, EF Terwilliger, and LJ Young (2001). "Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole". Neuroscience 21 (18): 7392–7396. PMID 11549749. http://www.ncbi.nlm.nih.gov/pubmed/11549749?itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum&ordinalpos=2.
- ↑ Vander, Arthur J. (1995). Renal physiology (5th ed.). New York: McGraw-Hill, Health Professions Division. ISBN 0-07-067009-9.
- ↑ Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ (March 2004). "Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice". Neuropsychopharmacology 29 (3): 483–93. doi:10.1038/sj.npp.1300360. PMID 14647484.
- ↑ Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS (August 2007). "Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression". Genes, Brain and Behavior 6 (6): 540–51. doi:10.1111/j.1601-183X.2006.00281.x. PMID 17083331.
- ↑ Lolait SJ, Stewart LQ, Jessop DS, Young WS, O'Carroll AM (February 2007). "The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors". Endocrinology 148 (2): 849–56. doi:10.1210/en.2006-1309. PMID 17122081.
- ↑ Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O'Carroll AM, Young WS (December 2004). "Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task". Horm Behav 46 (5): 638–45. doi:10.1016/j.yhbeh.2004.07.004. PMID 15555506.
- ↑ 11.0 11.1 Kanwar S, Woodman RC, Poon MC, Murohara T, Lefer AM, Davenpeck KL, Kubes P (1 October 1995). "Desmopressin induces endothelial P-selectin expression and leukocyte rolling in postcapillary venules". Blood 86 (7): 2760–6. PMID 7545469. http://bloodjournal.hematologylibrary.org/cgi/reprint/86/7/2760.
- ↑ Kaufmann JE, Oksche A, Wollheim CB, Günther G, Rosenthal W, Vischer UM (July 2000). "Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP". J. Clin. Invest. 106 (1): 107–16. doi:10.1172/JCI9516. PMID 10880054.
- ↑ Acher R, Chauvet J (July 1995). "The neurohypophysial endocrine regulatory cascade: precursors, mediators, receptors, and effectors". Front Neuroendocrinol 16 (3): 237–89. doi:10.1006/frne.1995.1009. PMID 7556852.
- ↑ Baum S, Nusbaum M, Tumen HJ (1970). "The control of gastrointestinal hemorrhage by selective mesenteric infusion of pitressin". Gastroenterology 58: 926.
- ↑ Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH (January 2004). "A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation". N. Engl. J. Med. 350 (2): 105–13. doi:10.1056/NEJMoa025431. PMID 14711909.
- ↑ Grmec S, Mally S (February 2006). "AVP improves outcome in out-of-hospital cardiopulmonary resuscitation of ventricular fibrillation and pulseless ventricular tachycardia: a observational cohort study". Crit Care 10 (1): R13. doi:10.1186/cc3967. PMID 16420660.
- ↑ Gueugniaud PY, David JS, Chanzy E, et al. (July 2008). "Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation". N. Engl. J. Med. 359 (1): 21–30. doi:10.1056/NEJMoa0706873. PMID 18596271.
- ↑ Palm C, Pistrosch F, Herbrig K, Gross P (July 2006). "Vasopressin antagonists as aquaretic agents for the treatment of hyponatremia". Am. J. Med. 119 (7 Suppl 1): S87–92. doi:10.1016/j.amjmed.2006.05.014. PMID 16843091. http://linkinghub.elsevier.com/retrieve/pii/S0002-9343(06)00549-3.
Further reading
- Rector, Floyd C.; Brenner, Barry M. (2004). Brenner & Rector's the kidney (7th ed.). Philadelphia: Saunders. ISBN 0-7216-0164-2. http://home.mdconsult.com/das/search/openres/56203699-5?searchisbn=460046813.
External links
- Molecular neurobiology of social bonding: Implications for autism spectrum disorders a lecture by Prof. Larry Young, Jan. 4, 2010.
|
|||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||