Anthocyanin


- Not to be confused with Anthocyanidin, their sugar free counterparts.
Anthocyanins (from Greek: ἀνθός (anthos) = flower + κυανός (kyanos) = blue) are water-soluble vacuolar pigments that may appear red, purple, or blue according to pH. They belong to a parent class of molecules called flavonoids synthesized via the phenylpropanoid pathway; they are odorless and nearly flavorless, contributing to taste as a moderately astringent sensation. Anthocyanins occur in all tissues of higher plants, including leaves, stems, roots, flowers, and fruits. Anthoxanthins are their clear, white to yellow counterparts occurring in plants. Anthocyanins are derivatives of anthocyanidins which include pendant sugars.
Contents |
Function

In flowers, bright reds and purples are adaptive for attracting pollinators. In fruits, the colorful skins also attract the attention of animals, which may eat the fruits and disperse the seeds. In photosynthetic tissues (such as leaves and sometimes stems), anthocyanins have been shown to act as a "sunscreen", protecting cells from high-light damage by absorbing blue-green and UV light, thereby protecting the tissues from photoinhibition, or high-light stress. This has been shown to occur in red juvenile leaves, autumn leaves, and broad-leaved evergreen leaves that turn red during the winter. It has also been proposed that red coloration of leaves may camouflage leaves from herbivores blind to red wavelengths, or signal unpalatability, since anthocyanin synthesis often coincides with synthesis of unpalatable phenolic compounds.[1]
In addition to their role as light-attenuators, anthocyanins also act as powerful antioxidants. However, it is not clear whether anthocyanins can significantly contribute to scavenging of free-radicals produced through metabolic processes in leaves, since they are located in the vacuole, and thus, spatially separated from metabolic reactive oxygen species. Some studies have shown that hydrogen peroxide produced in other organelles can be neutralized by vacuolar anthocyanin.
Occurrence

| Food source | Anthocyanin content in mg per 100 g |
|---|---|
| açaí | 320 |
| blackcurrant | 190-270 |
| chokeberry | 1,480[2] |
| eggplant | 750 |
| orange | ~200 |
| Marion blackberry | 317[3] |
| black raspberry | 589[4] |
| raspberry | 365 |
| wild blueberry | 558[5] |
| cherry | 350-400 |
| redcurrant | 80-420 |
| red grape | 888[6] |
| red wine | 24-35 |
| purple corn | 1,642[7] |
Anatomically, anthocyanins are found in the cell vacuole, mostly in flowers and fruits but also in leaves, stems, and roots. In these parts they are found predominantly in outer cell layers such as the epidermis and peripheral mesophyll cells.
Most frequent in nature are the glycosides of cyanidin, delphinidin, malvidin, pelargonidin, peonidin and petunidin. Roughly 2% of all hydrocarbons fixated in photosynthesis are converted into flavonoids and their derivatives such as the anthocyanins. There is no less than 109 tons of anthocyanins produced in nature per year. Not all land plants contain anthocyanin; in the Caryophyllales (including cactus, beets, and amaranth), they are replaced by betalains.
Plants rich in anthocyanins are Vaccinium species, such as blueberry, cranberry and bilberry, Rubus berries including black raspberry, red raspberry and blackberry, blackcurrant, cherry, eggplant peel, black rice, Concord grape and muscadine grape, red cabbage and violet petals. Anthocyanins are less abundant in banana, asparagus, pea, fennel, pear and potato, and may be totally absent in certain cultivars of green gooseberries.[2]
The highest recorded amount appears to be specifically in the seed coat of black soybean (Glycine max L. Merr.) containing some 2,000 mg per 100 g[8] and in skins and pulp of black chokeberry (Aronia melanocarpa L.) (table). However, the Amazonian palmberry, açaí, having about 320 mg per 100 g[9] of which cyanidin-3-glucoside is the most prevalent individual anthocyanin (approximately 10 mg per 100 g),[10] is also a high-content source for which only a small fraction of total anthocyanins has been determined to date. Due to critical differences in sample origin, preparation and extraction methods determining anthocyanin content,[11][12] the values presented in the adjoining table are not directly comparable.
Nature, primitive agriculture, and plant breeding have produced various uncommon crops containing anthocyanins, including blue- or red-fleshed potatoes and purple or red broccoli, cabbage, cauliflower, carrots and corn. Tomatoes have been bred conventionally for high anthocyanin content by crossing wild relatives with the common tomato to transfer a gene called the anthocyanin fruit tomato ("aft") gene into a larger and more palatable fruit.[13]
Tomatoes have also been genetically modified with transcription factors from snapdragons to produce high levels of anthocyanins in the fruits.[14][15][16] Anthocyanins can also be found in naturally ripened olives,[17][18] and are partly responsible for the red and purple colors of some olives.[17]
Autumn leaf color

Plants with abnormally high anthocyanin quantities are popular as ornamental plants. Many science textbooks incompletely state that autumn coloration (including red) is the result of breakdown of green chlorophyll, which unmasks the already-present orange, yellow, and red pigments (carotenoids, xanthophylls, and anthocyanins, respectively). While this is indeed the case for the carotenoids and xanthophylls (orange and yellow pigments), anthocyanins are not synthesized until the plant has begun breaking down the chlorophyll, presumably for photoprotection during nitrogen translocation.
Structure
Anthocyanidins: Flavylium cation derivatives
See Anthocyanidins article.
| Anthocyanidin | Basic structure | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|---|
| Aurantinidin | 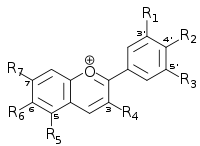 |
−H | −OH | −H | −OH | −OH | −OH | −OH |
| Cyanidin | −OH | −OH | −H | −OH | −OH | −H | −OH | |
| Delphinidin | −OH | −OH | −OH | −OH | −OH | −H | −OH | |
| Europinidin | −OCH3 | −OH | −OH | −OH | −OCH3 | −H | −OH | |
| Luteolinidin | −OH | −OH | −H | −H | −OH | −H | −OH | |
| Pelargonidin | −H | −OH | −H | −OH | −OH | −H | −OH | |
| Malvidin | −OCH3 | −OH | −OCH3 | −OH | −OH | −H | −OH | |
| Peonidin | −OCH3 | −OH | −H | −OH | −OH | −H | −OH | |
| Petunidin | −OH | −OH | −OCH3 | −OH | −OH | −H | −OH | |
| Rosinidin | −OCH3 | −OH | −H | −OH | −OH | −H | −OCH3 |
Anthocyanins: Glucosides of anthocyanidins
The anthocyanins, anthocyanidins with sugar group(s), are mostly 3-glucosides of the anthocyanidins. The anthocyanins are subdivided into the sugar-free anthocyanidin aglycones and the anthocyanin glycosides. As of 2003 more than 400 anthocyanins had been reported[19] while more recent literature (early 2006), puts the number at more than 550 different anthocyanins. The difference in chemical structure that occurs in response to changes in pH is the reason why anthocyanins are often used as pH indicator, as they change from red in acids to blue in bases.
Biosynthesis

- Anthocyanin pigments are assembled like all other flavonoids from two different streams of chemical raw materials in the cell:
- One stream involves the shikimate pathway to produce the amino acid phenylalanine. (see phenylpropanoids)
- The other stream produces 3 molecules of malonyl-CoA, a C3 unit from a C2 unit (acetyl-CoA).[20]
- These streams meet and are coupled together by the enzyme chalcone synthase (CHS), which forms an intermediate chalcone via a polyketide folding mechanism that is commonly found in plants.
- The chalcone is subsequently isomerized by the enzyme chalcone isomerase (CHI) to the prototype pigment naringenin.
- Naringenin is subsequently oxidized by enzymes such as flavanone hydroxylase (FHT or F3H), flavonoid 3' hydroxylase and flavonoid 3' 5'-hydroxylase.
- These oxidation products are further reduced by the enzyme dihydroflavonol 4-reductase (DFR) to the corresponding colorless[21] leucoanthocyanidins.
- It was believed that leucoanthocyanidins are the immediate precursors of the next enzyme, a dioxygenase referred to as anthocyanidin synthase (ANS) or leucoanthocyanidin dioxygenase (LDOX). It was recently shown however that flavan-3-ols, the products of leucoanthocyanidin reductase (LAR), are the true substrates of ANS/LDOX.
- The resulting, unstable anthocyanidins are further coupled to sugar molecules by enzymes like UDP-3-O-glucosyltransferase[22] to yield the final relatively stable anthocyanins.
More than five enzymes are thus required to synthesize these pigments, each working in concert. Any even minor disruption in any of the mechanism of these enzymes by either genetic or environmental factors would halt anthocyanin production.
Potential food value
Anthocyanins are considered secondary metabolites as a food additive with E number 163.
Although anthocyanins are powerful antioxidants in vitro,[23] it is unlikely this antioxidant property is conserved after the plant which produced the anthocyanins is consumed. As interpreted by the Linus Pauling Institute and European Food Safety Authority, dietary anthocyanins and other flavonoids have little or no direct antioxidant food value following digestion.[24][25][26] Unlike controlled test tube conditions, the fate of anthocyanins in vivo shows they are poorly conserved (less than 5%), with most of what is absorbed existing as chemically-modified metabolites which become rapidly excreted.[27]
The increase in antioxidant capacity of blood seen after the consumption of anthocyanin-rich foods may not be caused directly by the anthocyanins, but instead may result from increased uric acid levels derived from metabolism of flavonoids.[27]
Dye-sensitized solar cells
Anthocyanins are being used in organic solar cells because of their ability to absorb light and convert it into electrons.[28] There are many benefits to using dye-sensitized solar cells instead of the traditional silicon cells, such as abundance of anthocyanins, the projected 90% efficiency, and the ability to bend or print inks used in newer manufacturing processes.[29]
Research
Richly concentrated as pigments in berries, anthocyanins were the topics of research presented at a 2007 symposium on health benefits that may result from berry consumption.[30] Laboratory-based evidence was provided for potential health effects against:
- cancer
- aging and neurological diseases
- inflammation
- diabetes
- bacterial infections
Cancer research on anthocyanins is the most advanced, where black raspberry (Rubus occidentalis L.) preparations were first used to inhibit chemically induced cancer of the rat esophagus by 30-60% and of the colon by up to 80%.[30][31] Effective at both the initiation and promotion/progression stages of tumor development, black raspberries are a practical research tool and a promising therapeutic source, as they contain the richest contents of anthocyanins among native North American Rubus berries.[4]
Work on laboratory cancer models has shown that black raspberry anthocyanins inhibit promotion and progression of tumor cells by
- stalling growth of pre-malignant cells
- accelerating the rate of cell turnover, called apoptosis, effectively making the cancer cells die faster
- reducing inflammatory mediators that initiate tumor onset
- inhibiting growth of new blood vessels that nourish tumors, a process called angiogenesis
- minimizing cancer-induced DNA damage.
On a molecular level, berry anthocyanins were shown to turn off genes involved with proliferation, inflammation and angiogenesis.[32][33][34], while switching on apoptosis.[35][36]
In 2007, black raspberry studies entered the next pivotal level of research – the human clinical trial – for which several approved studies are underway to examine anti-cancer effects of black raspberries and cranberries on tumors in the esophagus, prostate and colon.[37]
Anthocyanins also fluoresce; combined with their antioxidant properties this can be a powerful tool for plant cell research, allowing live cell imaging for extended periods of time without a requirement for other fluorophores.[38]
See also
- Phenolic compounds in wine
References
- ↑ Jack Sullivan (1998). "Anthocyanin" (htm). Carnivorous Plant Newsletter (CPN) September 1998. http://www.carnivorousplants.org/cpn/samples/Science273anthocyanin.htm. Retrieved 2009-10-06.
- ↑ 2.0 2.1 Wu X, Gu L, Prior RL, McKay S (December 2004). "Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity". Journal of Agricultural and Food Chemistry 52 (26): 7846–56. doi:10.1021/jf0486850. PMID 15612766.
- ↑ Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB (December 2004). "Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties". Journal of Agricultural and Food Chemistry 52 (26): 8021–30. doi:10.1021/jf048619y. PMID 15612791.
- ↑ 4.0 4.1 Wada L, Ou B (June 2002). "Antioxidant activity and phenolic content of Oregon caneberries". Journal of Agricultural and Food Chemistry 50 (12): 3495–500. doi:10.1021/jf011405l. PMID 12033817.
- ↑ Hosseinian FS, Beta T (December 2007). "Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries". Journal of Agricultural and Food Chemistry 55 (26): 10832–8. doi:10.1021/jf072529m. PMID 18052240.
- ↑ Muñoz-Espada AC, Wood KV, Bordelon B, Watkins BA (November 2004). "Anthocyanin quantification and radical scavenging capacity of Concord, Norton, and Marechal Foch grapes and wines". Journal of Agricultural and Food Chemistry 52 (22): 6779–86. doi:10.1021/jf040087y. PMID 15506816.
- ↑ Lieberman S (2007). "The antioxidant power of purple corn: a research review". Alternative & Complementary Therapies 13 (2): 107–110. doi:10.1089/act.2007.13210.
- ↑ Choung MG, Baek IY, Kang ST, et al. (December 2001). "Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.)". J. Agric. Food Chem. 49 (12): 5848–51. doi:10.1021/jf010550w. PMID 11743773.
- ↑ Schauss AG, Wu X, Prior RL, et al. (November 2006). "Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae mart. (acai)". Journal of Agricultural and Food Chemistry 54 (22): 8598–603. doi:10.1021/jf060976g. PMID 17061839.
- ↑ Del Pozo-Insfran D, Brenes CH, Talcott ST (March 2004). "Phytochemical composition and pigment stability of Açai (Euterpe oleracea Mart.)". Journal of Agricultural and Food Chemistry 52 (6): 1539–45. doi:10.1021/jf035189n. PMID 15030208.
- ↑ Krenn L, Steitz M, Schlicht C, Kurth H, Gaedcke F (November 2007). "Anthocyanin- and proanthocyanidin-rich extracts of berries in food supplements--analysis with problems". Pharmazie 62 (11): 803-12. PMID 18065095.
- ↑ Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB (December 2004). "Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties". J Agric Food Chem 52 (26): 8021-30. PMID 15612791.
- ↑ Jones CM, Mes P, Myers JR (2003). "Characterization and inheritance of the Anthocyanin fruit (Aft) tomato". The Journal of Heredity 94 (6): 449–56. doi:10.1093/jhered/esg093. PMID 14691311.
- ↑ Butelli E, Titta L, Giorgio M, et al. (November 2008). "Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors". Nature Biotechnology 26 (11): 1301–8. doi:10.1038/nbt.1506. PMID 18953354.
- ↑ Purple tomato 'may boost health', Health, BBC News online, 26 October 2008
- ↑ Purple as a tomato: towards high anthocyanin tomatoes
- ↑ 17.0 17.1 Agati G, Pinelli P, Cortés Ebner S, Romani A, Cartelat A, Cerovic ZG (March 2005). "Nondestructive evaluation of anthocyanins in olive (Olea europaea) fruits by in situ chlorophyll fluorescence spectroscopy". Journal of Agricultural and Food Chemistry 53 (5): 1354–63. doi:10.1021/jf048381d. PMID 15740006.
- ↑ Stan Kailis, David Harris. Producing Table Olives. Landlinks Press.
- ↑ Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (November 2003). "Analysis and biological activities of anthocyanins". Phytochemistry 64 (5): 923–33. doi:10.1016/S0031-9422(03)00438-2. PMID 14561507.
- ↑ Jack Sullivan (1998). "Anthocyanin" (htm). Carnivorous Plant Newsletter (CPN) September 1998. http://www.carnivorousplants.org/cpn/samples/Science273anthocyanin.htm. Retrieved 2009-10-06.
- ↑ Nakajima J, Tanaka Y, Yamazaki M, Saito K (July 2001). "Reaction mechanism from leucoanthocyanidin to anthocyanidin 3-glucoside, a key reaction for coloring in anthocyanin biosynthesis". The Journal of Biological Chemistry 276 (28): 25797–803. doi:10.1074/jbc.M100744200. PMID 11316805.
- ↑ Kovinich N, Saleem A, Arnason JT, Miki B (2010). "Functional characterization of a UDP-glucose:flavonoid 3-O-glucosyltransferase from the seed coat of black soybean (Glycine max (L.) Merr.)". Phytochemistry. doi:10.1016/j.phytochem.2010.05.009.
- ↑ De Rosso VV, Morán Vieyra FE, Mercadante AZ, Borsarelli CD (October 2008). "Singlet oxygen quenching by anthocyanin's flavylium cations". Free Radical Research 42 (10): 885–91. doi:10.1080/10715760802506349. PMID 18985487.
- ↑ Lotito SB, Frei B (2006). "Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon?". Free Radic. Biol. Med. 41 (12): 1727–46. doi:10.1016/j.freeradbiomed.2006.04.033. PMID 17157175.
- ↑ Williams RJ, Spencer JP, Rice-Evans C (April 2004). "Flavonoids: antioxidants or signalling molecules?". Free Radical Biology & Medicine 36 (7): 838–49. doi:10.1016/j.freeradbiomed.2004.01.001. PMID 15019969.
- ↑ Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/20061, EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA)2, 3 European Food Safety Authority (EFSA), Parma, Italy, EFSA Journal 2010; 8(2):1489
- ↑ 27.0 27.1 "Studies force new view on biology of flavonoids", by David Stauth, EurekAlert!. Adapted from a news release issued by Oregon State University
- ↑ Cherepy, Nerine J.; Smestad, Greg P.; Grätzel, Michael; Zhang, Jin Z. (1997). "Ultrafast Electron Injection: Implications for a Photoelectrochemical Cell Utilizing an Anthocyanin Dye-Sensitized TiO2 Nanocrystalline Electrode". The Journal of Physical Chemistry B 101 (45): 9342–51. doi:10.1021/jp972197w. http://solideas.com/papers/JPhysChemB.pdf.
- ↑ Grätzel, Michael (October 2003). "Dye-sensitized solar cells". Journal of Photochemistry and Photobiology 4 (2): 145–53. doi:10.1016/S1389-5567(03)00026-1.
- ↑ 30.0 30.1 Journal of Agricultural and Food Chemistry Presents Research from the 2007 International Berry Health Benefits Symposium, Journal of Agricultural and Food Chemistry ACS Publications, February 2008
- ↑ Stoner GD. Black raspberries show multiple defenses in thwarting cancer, The Ohio State University Research News, undated
- ↑ Hou DX (March 2003). "Potential mechanisms of cancer chemoprevention by anthocyanins". Current Molecular Medicine 3 (2): 149–59. doi:10.2174/1566524033361555. PMID 12630561.
- ↑ Karlsen A, Retterstøl L, Laake P, et al. (August 2007). "Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults". The Journal of Nutrition 137 (8): 1951–4. PMID 17634269. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=17634269.
- ↑ Neto CC (June 2007). "Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases". Molecular Nutrition & Food Research 51 (6): 652–64. doi:10.1002/mnfr.200600279. PMID 17533651.
- ↑ Neto CC. (January 2007) Cranberry and its phytochemicals: a review of in vitro anticancer studies. J Nutr. 137(1 Suppl):186S-193S., [1]
- ↑ Thomasset S, Teller N, Cai H, Marko D, Berry DP, Steward WP, Gescher AJ. (June 2009). Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemother Pharmacol;64(1):201-11.PMID: 19294386 [2]
- ↑ Stoner GD, Wang LS, Zikri N, et al. (October 2007). "Cancer prevention with freeze-dried berries and berry components". Seminars in Cancer Biology 17 (5): 403–10. doi:10.1016/j.semcancer.2007.05.001. PMID 17574861.
- ↑ Wiltshire EJ, Collings DA (October 2009). "New dynamics in an old friend: dynamic tubular vacuoles radiate through the cortical cytoplasm of red onion epidermal cells". Plant & Cell Physiology 50 (10): 1826–39. doi:10.1093/pcp/pcp124. PMID 19762337.
External links
- Andersen, O.M. Flavonoids: Chemistry, Biochemistry and Applications. CRC Press, Boca Raton FL 2006.
- Robinson GM, Robinson R (1931). "A survey of anthocyanins. I". The Biochemical Journal 25 (5): 1687–705. PMID 16744735.
- Anthocyanin biosynthesis
- What do the traditional Maori diet, autumn leaves, and red wine have in common? on the Australian science series Catalyst.
- A discussion of the role of anthocyanins in hydrangea coloration
- Anthocyanins FAQ MadSci Network
- Lengthy discussion of Carnivorous Plants that in places touches on the function of anthocyanins in plants
- An excellent overview that provides useful info about the function & synthesis of anthocyanins. Also here is a modified draft.
- Kids orientented overview about leaves with anthocyanins on page 6, a curriculum supplement of Newspaper in Education (NIE) provided by The Washington Post.
|
||||||||||||||
|
||||||||||||||||||||