pH
- Acid dissociation constant
- Acid-base extraction
- Acid-base reaction
- Acid-base physiology
- Acid-base homeostasis
- Dissociation constant
- Acidity function
- Buffer solutions
- pH
- Proton affinity
- Self-ionization of water
- Acids:
- Lewis acids
- Mineral acids
- Organic acids
- Strong acids
- Superacids
- Weak acids
- Bases:
- Lewis bases
- Organic bases
- Strong bases
- Superbases
- Non-nucleophilic bases
- Weak bases
pH is a measure of the acidity or basicity of a solution. It is defined as the cologarithm of the activity of dissolved hydrogen ions (H+). Hydrogen ion activity coefficients cannot be measured experimentally, so they are based on theoretical calculations. The pH scale is not an absolute scale; it is relative to a set of standard solutions whose pH is established by international agreement.[1]
The concept of pH was first introduced by Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909. Sørensen suggested the notation "PH" for convenience, standing for "power of hydrogen",[2] using the cologarithm of the concentration of hydrogen ions in solution, p[H][3] Although this definition has been superseded p[H] can be measured if an electrode is calibrated with solution of known hydrogen ion concentration.
Pure water is said to be neutral. The pH for pure water at 25 °C is close to 7.0. Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are said to be basic or alkaline. pH measurements are important for medicine, biology, chemistry, food science, environmental science, oceanography and many other applications.
Contents |
Definitions
pH
pH is defined as minus the decimal logarithm of the hydrogen ion activity in an aqueous solution.[4] By virtue of its logarithmic nature, pH is a dimensionless quantity.
where aH is the (dimensionless) activity of hydrogen ions. The reason for this definition is that aH is a property of a single ion which can only be measured experimentally by means of an ion-selective electrode which responds, according to the Nernst equation, to hydogen ion activity. pH is commonly measured by means of a combined glass electrode, which measures the potential difference, or electromotive force, E, between an electrode sensitive to the hydrogen ion activity and a reference electrode, such as a calomel electrode or a silver chloride electrode. The combined glass electrode ideally follows the Nernst equation:
where E is a measured potential , E0 is the standard electrode potential, that is, the electode potential for the standard state in which the activity is one. R is the gas constant T is the temperature in Kelvin, F is the Faraday constant and n is the number of electrons transferred, one in this instance. The electrode potential, E, is proportional to the logarithm of the hydrogen ion activity.
This definition, by itself, is wholly impractical because the hydrogen ion activity is the product of the concentration and an activity coefficient. The single-ion activity coefficient of the hydrogen ion is a quantity which cannot be measured experimentally. To get round this difficulty the electrode is calibrated in terms of solutions of known activity.
The operational definition of pH is officially defined by International Standard ISO 31-8 as follows: [5] For a solution X, first measure the electromotive force EX of the galvanic cell
- reference electrode | concentrated solution of KCl || solution X | H2 | Pt
and then also measure the electromotive force ES of a galvanic cell that differs from the above one only by the replacement of the solution X of unknown pH, pH(X), by a solution S of a known standard pH, pH(S). The pH of X is then
The difference between the pH of solution X and the pH of the standard solution depends only on the difference between two measured potentials. Thus, pH is obtained from a potential measured with an electrode calibrated against one or more pH standards; a pH meter setting is adjusted such that the meter reading for a solution of a standard is equal to the value pH(S). Values pH(S) for a range of standard solutions S, along with further details, are given in the IUPAC recommendations.[6] The standard solutions are often described as standard buffer solution. In practice it is better to use two or more standard buffers to allow for small deviations from Nernst-law ideality in real electrodes. Note that because the temperature occurs in the defining equations, the pH of a solution is temperature-dependent.
Measurement of extremely low pH values, such as some very acidic mine waters,[7] requires special procedures. Calibration of the electrode in such cases can be done with standard solutions of concentrated sulfuric acid whose pH values can be calculated with using Pitzer parameters to calculate activity coefficients.[8]
pH is an example of an acidity function. Hydrogen ion concentrations can be measured in non-aqueous solvents, but this leads, in effect, to a different acidity function because the standard state for a non-aqueous solvent is different from the standard state for water. Superacids are a class of non-aqueous acids for which the Hammett acidity function, H0, has been developed.
p[H]
This was the original definition of Sørensen, [2] which was superseded in favour of pH. However, it is possible to measure the concentration of hydrogen ions directly, if the electrode is calibrated in terms of hydrogen ion concentrations. One way to do this, which has been used extensively, is to titrate a solution of known concentration of a strong acid with a solution of known concentration of strong alkali in the presence of a relatively high concentration of background electrolyte. Since the concentrations of acid and alkali are known it is easy to calculate the concentration of hydrogen ions so that the measured potential can be correlated with concentrations. The calibration is usually carried out using a Gran plot.[9] The calibration yieds a value for the standard electrode potential, E0, and a slope factor, f, so that the Nernst equation in the form
can be used to derive hydrogen ion concentrations from experimental measurements of E. The slope factor is usually slightly less than one. A slope factor of less than 0.95 indicates that the electrode is not functioning correctly. The presence of background electrolyte ensures that the hydrogen ion activity coefficient is effectively constant during the titration. As it is constant its value can be set to one by defining the standard state as being the solution containing the background electrolyte. Thus, the effect of using this procedure is to make activity equal to the numerical value of concentration.
The difference between p[H] and pH is quite small. It has been stated[10] that pH = p[H] + 0.04. Unfortunately it is common practice to use the term "pH" for both types of measurement.
pOH
pOH is sometimes used as a measure of the concentration of hydroxide ions, OH−, or alkalinity. pOH is not measured independently, but is derived from pH. The concentration of hydroxide ions in water is related to the concentration of hydrogen ions by
- [OH-] = KW /[H+]
where KW is the self-ionisation constant of water. Taking cologarithms
- pOH = pKW - pH.
So, at room temperature pOH ≈ 14 - pH. However this relationship is not strictly valid in other circumstances, such as in measurements of soil alkalinity.
Applications

Pure water has a pH around 7; the exact values depends on the temperature. When an acid is dissolved in water the pH will be less than 7 and and when a base, or alkali is dissolved in water the pH will be greater than 7. A solution of a strong acid, such as hydrochloric acid, at concentration 1 mol dm-3 has a pH of 0. A solution of a strong alkali, such as sodium hydroxide, at concentration 1 mol dm-3 has a pH of 14. Thus, measured pH values will mostly lie in the range 0 to 14. Since pH is a logarithmic scale a difference of one pH unit is equivalent to a ten-fold difference in hydrogen ion concentration.
Because the glass electrode (and other ion selective electrodes) reponds to activity, the electrode should be calibrated in a medium similar to the one being investigated. For instance, if one wishes to measure the pH of a seawater sample, the electrode should be calibrated in a solution resembling seawater in its chemical composition, as detailed below.
An approximate measure of pH may be obtained by using a pH indicator. A pH indicator is a substance that changes colour around a particular pH value. It is a weak acid or weak base and the colour change occurs around 1 pH unit either side of its acid dissociation constant, or pKa, value. For example, the naturally occuring indicator litmus is red in acidic solutions (pH<7) and blue in alkaline (pH>7) solutions. Universal indicator consists of a mixture of indicators such that there is a continuous colour change from about pH 2 to pH 10. Universal indicator paper is simple paper that has been impregnated with universal indicator.
| Indicator | Low pH color | Transition pH range | High pH color |
|---|---|---|---|
| Thymol blue (first transition) | red | 1.2–2.8 | orange |
| Methyl red | red | 4.4–6.2 | yellow |
| Bromothymol blue | yellow | 6.0–7.6 | blue |
| Thymol blue (second transition) | yellow | 8.0–9.6 | blue |
| Phenolphthalein | colorless | 8.3–10.0 | purple |
A solution whose pH is 7 is said to be neutral, that is, it is neither acidic nor basic. Water is subject to a self-ionisation process.
- H2O
 H+ + OH−
H+ + OH−
The dissociation constant, KW, has a value of about 10-14, so in neutral solution of a salt both the hydrogen ion concentration and hydroxide ion concentration are about 10-7 mol dm-3. The pH of pure water decreases with increasing temperatures. For example, the pH of pure water at 50 °C is 6·55. Note, however, that water that has been exposed to air is mildly acidic. This is because water absorbs carbon dioxide from the air, and carbon dioxide is an acid. After absorption it is slowly converted into the weak acid, carbonic acid, which then dissociates to liberate hydrogen ions.
- CO2 + H2O
 H2CO3
H2CO3  HCO3− + H+
HCO3− + H+
The pH of distilled water at room temperature is about 5.7.
Calculation of pH for weak and strong acids
In the case of a strong acid, there is complete dissociation, so the pH is simply equal to minus the logarithm of the acid concentration. For example, a 0.01 molar solution of hydrochloric acid has a pH of −log(0.01), that is, pH = 2.
The pH of a solution of a weak acid may be calculated by means of an ICE table. For acids with a pKa value greater than about 2,
- pH = ½ ( pKa - log c0),
where c0 is the concentration of the acid. This is equivalent to Burrows' weak acid pH equation
A more general method is as follows. Consider the case of dissolving a weak acid, HA, in water. First write down the equilibrium expression.
- HA
 A- + H+
A- + H+
The equilibrium constant for this reaction is specified by
where [] indicates a concentration. The analytical concentration of the two reagents, CA for [A-] and CH for [H+] must be equal to the sum of concentrations of those species that contain the reagents. CH is the concentration of added mineral acid.
- CA = [A-] +Ka[A-][H+]
- CH = [H+] +Ka[A-][H+]
From the first equation
Substitution of this expression into the second equation gives
This simplifies to a quadratic equation in the hydrogen ion concentration
Solution of this equation gives [H+] and hence pH.
This method can also be used for polyprotic acids. For example, for the diprotic acid oxalic acid, writing A2- for the oxalate ion,
- CA = [A2-] + β1[A2-][H+]+ β2[A2-][H+]2
- CH = [H+] +β1[A2-][H+] + 2β2[A2-][H+]2
where β1 and β2 are cumulative protonation constants. Following the same procedure of substituting from the first equation into the second, a cubic equation in [H+] results. In general, the degree of the equation is one more than the number of ionisable protons. The solution of these equations can be obtained relatively easily with the aid of a spreadsheet such as EXCEL or Origin.
pH in nature
pH-dependent plant pigments that can be used as pH indicators occur in many plants, including hibiscus, marigold, red cabbage (anthocyanin)[11], and red wine.
Seawater
The pH of seawater is very important and there is evidence for ocean acidification. Distinct pH scales exist depending on the method used to calibrate the electrode.[12]
- Using standard buffers: The ionic strength of standard buffer solutions is much lower, at about 0.1 M, than that of seawater, which is about 0.7 M. Consequently they are not recommended for use in measuring the pH of seawater.
- A set of buffers based on artificial seawater was developed.[13] This pH scale is referred to as the total scale, denoted by pHT. The total scale was defined using a medium containing sulfate ions, which are subject to the proton absorbing equilibrium H+ + SO42−
 HSO4−.
HSO4−. - The free scale, denoted by pHF, omits the effect of sulfate ions and focuses solely on [H+]F, in principle making it a simpler representation of hydrogen ion concentration. Analytically, only [H+]T can be determined,[14] therefore, [H+]F must be estimated using the [SO42−] and the dissociation constant constant of HSO4−. The utility of this scale is limited by the complexity of the calculations. pH values measured on the the free scale differ by up to 0.12 pH units from both the total and seawater scales.
- The seawater scale, denoted by pHSWS , takes account of the fact that hydrogen fluoride is a weak acid, H+ + F−
 HF. However, the concentration of sulfate ions is about 400 times larger than the concentration of fluoride, so the difference between the total and seawater scales is very small.
HF. However, the concentration of sulfate ions is about 400 times larger than the concentration of fluoride, so the difference between the total and seawater scales is very small.
Living systems
| Compartment | pH |
|---|---|
| Gastric acid | 0.7 |
| Lysosomes | 4.5 |
| Granules of chromaffin cells | 5.5 |
| Urine | 6.0 |
| Neutral H2O at 37 °C | 6.81 |
| Cytosol | 7.2 |
| Cerebrospinal fluid (CSF) | 7.3 |
| Blood | 7.34 – 7.45 |
| Mitochondrial matrix | 7.5 |
| Pancreas secretions | 8.1 |
The pH of different cellular compartments, body fluids, and organs is usually tightly regulated in a process called acid-base homeostasis.
The pH of blood is usually slightly basic with a value of pH 7.4. This value is often referred to as physiological pH in biology and medicine.
Plaque can create a local acidic environment that can result in tooth decay by demineralization.
Enzymes and other proteins have an optimum pH range and can became inactivated or denatured outside this range.
See also
- Acidosis
- Alkalosis
References
- ↑ "The Measurement of pH - Definition, Standards and Procedures – Report of the Working Party on pH, IUPAC Provisional Recommendation]" (2001). A proposal to revise the current IUPAC 1985 and ISO 31-8 definition of pH.
- ↑ 2.0 2.1 Carlsberg Group Company History Page, http://www.carlsberggroup.com/Company/Research/Pages/pHValue.aspx
- ↑ Sørensen, http://www.geocities.com/bioelectrochemistry/sorensen.htm
- ↑ "pH". IUPAC Goldbook.
- ↑ Quantities and units – Part 8: Physical chemistry and molecular physics, Annex C (normative): pH. International Organization for Standardization, 1992.
- ↑ Definitions of pH scales, standard reference values, measurement of pH, and related terminology. Pure Appl. Chem. (1985), 57, pp 531–542.
- ↑ Nordstrom, DK et al (2000) Negative pH and extremely acidic mine waters from Iron Mountain California. Environ Sci Technol,34, 254-258.
- ↑ Zemaitis, J.F.; Clark, D.M; Rafal, M; Scrivner, N.C. (1986). Handbook of Aqueous Electrolyte Thermodynamics: Theory & Application. Wiley. ISBN 978-0-8169-0350-4. Chapter 4
- ↑ Rossotti, F.J.C.; Rossotti, H. (1965). "Potentiometric titrations using Gran plots: A textbook omission". J. Chem. Ed. 42: 375–378.
- ↑ Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M.J.K.; Denney, R. C.; Thomas, M. J. K. Vogel's Quantitative Chemical Analysis (6th Edn.) New York:Prentice Hall. ISBN 0-582-22628-7. Section 13.23, "Determination of pH"
- ↑ chemistry.about.com
- ↑ Zeebe, R.E.; Wolf-Gladrow, D. (2001). CO2 in seawater: equilibrium, kinetics, isotopes. Elsevier. ISBN 0 444 50946 1.
- ↑ Hansson, I (1973). "A new set of pH-scales and standard buffers for seawater". Deep Sea Research 20: 479–491. doi:.
- ↑ Dickson, A. G. (1984). "pH scales and proton-transfer reactions in saline media such as sea water". Geochim. Cosmochim. Acta 48: 2299–2308. doi:.
- ↑ Boron, Walter, F.; Boulpaep, E.L. (2004). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 1-4160-2328-3.
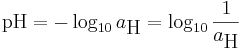
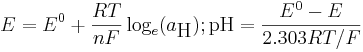
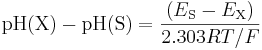
![E = E^0 + f\frac{RT}{nF} \log_e[\mbox{H}^+]](/2009-wikipedia_en_wp1-0.7_2009-05/I/10665ee41eae75113e529058df52a5a1.png)
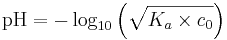
![K_\text{a}=\mathrm{\frac{[A^-][H^+]}{[HA]}}](/2009-wikipedia_en_wp1-0.7_2009-05/I/155bb435b85f9ce7b514b9f7e5a1d685.png)
![\mathrm{[A^-]=\frac{\mathit C_A}{1+\mathit K_a[H^+]}}](/2009-wikipedia_en_wp1-0.7_2009-05/I/89ba28acaca0ef565373f502d4c0af57.png)
![\mathrm{\mathit C_ H=[H^+] + \frac{\mathit K_a \mathit C_A [H^+]}{1+\mathit K_a [H^+]}}](/2009-wikipedia_en_wp1-0.7_2009-05/I/e5611b786a689a1d2efc8aa0a3d0224c.png)
![\mathrm{\mathit K_a[H^+]^2 + \bigg(1+(\mathit C_A-\mathit C_H)\mathit K_a \bigg)[H^+] -\mathit C_H = 0}](/2009-wikipedia_en_wp1-0.7_2009-05/I/85a759727abb7d98515adfc61a32af7b.png)
