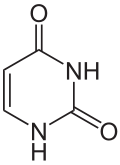
Uracil
| Uracil | |
|---|---|
 |
|
| IUPAC name | Pyrimidine-2,4(1H,3H)-dione |
| Other names | Uracil, 2-oxy-4-oxy pyrimidine, 2,4(1H,3H)-pyrimidinedione, 2,4-dihydroxypryimidine, 2,4-pyrimidinediol |
| Identifiers | |
| CAS number | 66-22-8 |
| RTECS number | YQ8650000 |
| SMILES |
|
| Properties | |
| Molecular formula | C4H4N2O2 |
| Molar mass | 112.08676 g/mol |
| Appearance | Solid |
| Melting point |
335 °C |
| Boiling point |
N/A |
| Solubility in water | Soluble |
| Hazards | |
| Main hazards | carcinogen & teratogen with chronic exposure |
| NFPA 704 |
 1
1
0
|
| Flash point | non flammable |
| Related compounds | |
| Related compounds | Thymine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Uracil is a common and naturally occurring pyrimidine derivative.[1] Originally discovered in 1900, it was isolated by hydrolysis of yeast nuclein that was found in bovine thymus and spleen, herring, sperm, and wheat germ.[2] It is a planar, unsaturated compound that has the ability to absorb light.[3]
Contents |
Properties
Found in RNA, it base pairs with adenine and is replaced by thymine in DNA. Methylation of uracil produces thymine.[4] It turns into thymine to protect the DNA and to improve the efficiency of DNA replication. Uracil can base pair with any of the bases depending on how the molecule arranges itself on the helix, but readily pairs with adenine because the methyl group is repelled into a fixed position.[4] Uracil pairs with adenine through hydrogen bonding. Uracil is the hydrogen bond acceptor and can form two hydrogen bonds. Uracil can also bind with a ribose sugar to form a ribonucleoside, uridine. When a phosphate attaches to uridine, uridine 5'-monophosphate is produced.[3]
Uracil undergoes amide-imidic acid tautomeric shifts because any nuclear instability the molecule may have from the lack of formal aromaticity is compensated by the cyclic-amidic stability.[2] The keto tautomer is referred to the lactam structure, while the imidic acid tautomer is referred to as the lactim structure. These tautomeric forms are predominant at pH=7. The lactam structure is the most common form of uracil.
-
 Uracil tautomers: Keto or lactam structure (left) and imidic or lactim structure (right)
Uracil tautomers: Keto or lactam structure (left) and imidic or lactim structure (right)
Uracil also recycles itself to form nucleotides by undergoing a series of phosphoribosyltransferase reactions.[1] Degradation of uracil produces substrates, aspartate, carbon dioxide, and ammonia.[1]
- C4H4N2O2 → H3NCH2CH2COO- + NH4 + CO2
Oxidative degradation of uracil produces urea and maleic acid in the presence of H2O2 and Fe2+ or in the presence of diatomic oxygen and Fe2+.
Uracil is a weak acid, the first site of ionization of uracil is not known.[5] The negative charge is placed on the oxygen anion and produces a pKa of less than or equal to 12. The basic pKa = -3.4, while the acidic pKa = 9.389. In the gas phase, uracil has 4 sites that are more acidic than water.[6]
Synthesis
There are many laboratory syntheses of uracil available. The first reaction is the simplest of the syntheses, by adding water to cytosine to produce uracil and ammonia.[1] The most common way to synthesize uracil is by the condensation of malic acid with urea in fuming sulfuric acid[2] as seen below also. Uracil can also be synthesized by a double decomposition of thiouracil in aqueous chloroacetic acid.[2]
- C4H5N3O + H2O → C4H4N2O2 + NH3
- C4H4O4 + CH4N2O → C4H4N2O2 + 2 H2O + CO
Photodehydrogenation of 5,6-diuracil, which is synthesized by beta-alanine reacting with urea, produces uracil.[7]
Reactions
Uracil readily undergoes regular reactions including oxidation, nitration, and alkylation. While in the presence of PhOH/NaOCl, uracil can be visualized in the blue region of UV light.[2] Uracil also has the capability to react with elemental halogens because of the presence of more than one strongly electron donating group.[2]
Uracil readily undergoes addition to ribose sugars and phosphates to partake in synthesis and further reactions in the body. Uracil becomes uridine, uridine monophosphate (UMP), uridine diphosphate (UDP), uridine triphosphate (UTP), and uridine diphosphate glucose (UDP-glucose). Each one of these molecules in synthesized in the body and has specific functions.
-
 Chemical structure of uridine
Chemical structure of uridine
When uracil reactes with anhydrous hydrazine a first order kinetic reaction occurs and the ring of uracil opens up.[8] If the pH of the reaction increases to >10.5 the uracil anion forms making the reaction go much slower, the same slowing of the reaction occurs if the pH decreases because of the protonation of the hydrazine.[8] The reactivity of uracil is unchanged even if the temperature changes.[8]
Uses
Uracil can be used for drug delivery and as a pharmaceutical. When elemental fluorine is reacted with uracil, 5-fluorouracil is produced. 5-Fluorouracil is an anticancer drug (antimetabolite) used to masquerade as uracil during the nucleic acid replication process.[1] Because 5-Fluorouracil is similar in shape to, but does not perform the same chemisty as uracil the drug inhibits RNA replication enzymes, thereby eliminating RNA synthesis and stopping the growth of cancerous cells.[1]
Uracil's use in the body is to help carry out the synthesis of many enzymes necessary for cell function through bonding with riboses and phosphates.[1] Uracil serves as allosteric regulator and coenzyme for reactions in the human body and in plants.[9] UMP controls the activity of carbamoyl phosphate synthetase and aspartate transcarbamoylase in plants, while UDP and UTP requlate CPSase II activity in animals. UDP-glucose regulates the conversion of glucose to galactose in the liver and other tissues in the process of carbohydrate metabolism.[9] Uracil is also involved in the biosynthesis of polysaccharides and the transportation of sugars containing aldehydes.[9]
It can also increase the risk for cancer in cases where the body is extremely deficient in folate.[10] The deficiency in folate leads to increased ratio of deoxyuracilmonophosphates (dUMP)/deoxythyminemonophosphates (dTMP) and uracil misincorporation into DNA and eventually low production of DNA.[10]
Uracil can be used to determine microbial contamination of tomatoes. The presence of uracil is an indication of lactic acid bacteria contamination in the fruit.[11] Uracil derivatives containing a diazine ring are used in pesticides.[12] Uracil derivatives are more often used as antiphotosynthetic herbicides, destroying weeds in cotton, sugar beet, turnips, soya, peas, sunflower crops, vineyards, berry plantations, and orchards.[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Garrett, Reginald H.; Grisham, Charles M. Principals of Biochemistry with a Human Focus. United States: Brooks/Cole Thomson Learning, 1997.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Brown, D.J. Heterocyclic Compounds: Thy Pyrimidines. Vol 52. New York: Interscience, 1994.
- ↑ 3.0 3.1 Horton, Robert H.; et al.Principles of Biochemistry. 3rd ed. Upper Saddle River, NJ: Prentice Hall, 2002.
- ↑ 4.0 4.1 www.madsci.org
- ↑ Zorbach, W.W. Synthetic Procedures in Nucleic Acid Chemistry: Physical and Physicochemical Aids in Determination of Structure. Vol 2. New York: Wiley-Interscience, 1973.
- ↑ Lee, J.K.; Kurinovich, Ma. J Am Soc Mass Spectrom.13(8), 2005, 985-95.
- ↑ Chittenden, G.J.F.; Schwartz, Alan W. Nature.263,(5575), 350-1.
- ↑ 8.0 8.1 8.2 Kochetkov, N.K. and Budovskii, E.I. Organic Chemistry of Nucleic Acids Part B. New York: Plenum Press, 1972.
- ↑ 9.0 9.1 9.2 Brown, E.G. Ring Nitrogen and Key Biomolecules: The Biochemistry of N-Heterocycles. Boston: Lluwer Academic Publishers, 1998.
- ↑ 10.0 10.1 Mashiyama, S.T; et al.'Anal Biochem. 330(1),2004, 58-69.
- ↑ Hildalgo, A; et al.'J Agric Food Chem.53(2),2005, 349-55.
- ↑ 12.0 12.1 Pozharskii, A.F.; et al.Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry and Biochemistry and the Role of Heterocycles in Science, Technology, Medicine, and Agriculture. New York: John Wiley and Sons, 1997.
|
|||||||||||||||||||||||||||