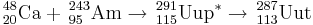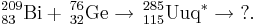Ununpentium
|
|||||||||||||||||||
| General | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununpentium, Uup, 115 | ||||||||||||||||||
| Element category | presumably poor metals | ||||||||||||||||||
| Group, Period, Block | 15, 7, p | ||||||||||||||||||
| Standard atomic weight | [288] g·mol−1 | ||||||||||||||||||
| Electron configuration | perhaps [Rn] 5f14 6d10 7s2 7p3 (guess based on bismuth) |
||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 5 | ||||||||||||||||||
| CAS registry number | 54085-64-2 | ||||||||||||||||||
| Most-stable isotopes | |||||||||||||||||||
|
|||||||||||||||||||
| References | |||||||||||||||||||
Ununpentium (pronounced /ˌjuːnənˈpɛntiəm/ or /ˌʌnənˈpɛntiəm/) is the temporary name of a synthetic superheavy element in the periodic table that has the temporary symbol Uup and has the atomic number 115.
Two isotopes are currently known, Uup-287 and Uup-288.
Element 115 also falls in the center of the theoretical island of stability. The most stable isotope of ununpentium is predicted to be Uup-299, containing the theorized "magic number" of 184 neutrons. The most neutron rich isotope to date is Uup-288, which contains only 173 neutrons.
Contents |
Discovery profile
On February 2, 2004, synthesis of ununpentium was reported in Physical Review C by a team composed of Russian scientists at the Joint Institute for Nuclear Research in Dubna, and American scientists at the Lawrence Livermore National Laboratory.[1][2] The team reported that they bombarded americium-243 with calcium-48 ions to produce four atoms of ununpentium. These atoms, they report, decayed by emission of alpha-particles to ununtrium in approximately 100 milliseconds.
The Dubna-Livermore collaboration has strengthened their claim for the discovery of ununpentium by conducting chemical experiments on the decay daughter 268Db. In experiments in June 2004 and December 2005, the Dubnium isotope was successfully identified by milking the Db fraction and measuring any SF activities.[3][4] Both the half-life and decay mode were confirmed for the proposed 268Db which lends support to the assignment of Z=115 to the parent nuclei.
Theoretical calculation in a quantum tunneling model supports the experimental alpha decay half-lives.[5]
Naming
Current names
The element with Z=115 is historically known as eka-bismuth. Ununpentium (Uup) is a temporary IUPAC systematic element name. Research scientists usually refer to the element simply as element 115 (E115).
Proposed names by claimants
Claims to the discovery of element 115 have been put forward by Dmitriev of the Dubna team. The Joint Working Party will decide to whom the right to suggest a name will be given. The IUPAC have the final say on the official adoption of a name. The table below gives the names that the teams above have suggested and which can be verified by press interviews.
Disallowed names
According to IUPAC rules, names used for previous elements that have ultimately not been adopted are not allowed to be proposed for future use. The table below summarises those names which are probably not allowed to be proposed by the claimant laboratories under the rules.
| Name | Symbol | Reason |
|---|---|---|
| Russium | Rs | Used for claimed discovery of element 43 |
| Kurchatovium | Ku | Used for claimed discovery of element 104 |
Electronic structure
Ununpentium has 6 full shells, 7s+5p+4d+2f=18 full subshells, and 115 orbitals:
Bohr model: 2, 8, 18, 32, 32, 18, 5
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f146d107p3
Extrapolated chemical properties of eka-bismuth
Oxidation states
Element 115 is projected to be the third member of the 7p series of non-metals and the heaviest member of group 15 (VA) in the Periodic Table, below bismuth. In this group, each member is known to portray the group oxidation state of +V but with differing stability. For nitrogen, the +V state is very difficult to achieve due to the lack of low-lying d-orbitals and the inability of the small nitrogen atom to accommodate five ligands. The +V state is well represented for phosphorus, arsenic, and antimony. However, for bismuth it is rare due to the reluctance of the 6s2 electron to participate in bonding. This effect is known as the "inert pair effect" and is commonly linked to relativistic stabilisation of the 6s-orbitals. It is expected that element 115 will continue this trend and portray only +III and +I oxidation states. Nitrogen(I) and bismuth(I) are known but rare and Uup(I) is likely to show some unique properties.[6]. B.L. Johnson supposes ununpentium to be dark metallic.
Chemistry
It is expected that the chemistry of ununpentium will be related to its lighter homologue bismuth. In this regard it is expected to undergo oxidation only as far as the trioxide Uup2O3. Oxidation with the more reactive halogens should form the trihalides, such as UupF3 and UupCl3. The less-oxidising, heavier halogens, may well only be able to promote the formation of the monohalides, UupBr and UupI.
History of synthesis of isotopes by hot fusion
238U(51V,xn)289−xUup
There are strong indications that this reaction was performed in late 2004 as part of a uranium(IV) fluoride target test at the GSI. No reports have been published suggesting that no products atoms were detected, as anticipated by the team.[7]
243Am(48Ca,xn)291−xUup (x=3,4)
This reaction was first performed by the team in Dubna in July-August 2003. In two separate runs they were able to detect 3 atoms of 288Uup and a single atom of 287Uup. The reaction was studied further in June 2004 in an attempt to isolate the descendant 268Db from the 288Uup decay chain. After chemical separation of a +4/+5 fraction, 15 SF decays were measured with a lifetime consistent with 268Db. In order to prove that the decays were from dubnium-268, the team repeated the reaction in August 2005 and separated the +4 and +5 fractions and further separated the +5 fractions into tantalum-like and niobium-like ones. Five SF activities were observed, all occurring in the +5 fractions and none in the tantalum-like fractions, proving that the product was indeed isotopes of dubnium.
Chronology of isotope discovery
| Isotope | Year discovered | Discoverer reaction |
|---|---|---|
| 287Uup | 2003 | 243Am(48Ca,4n) |
| 288Uup | 2003 | 243Am(48Ca,3n) |
Yields of isotopes
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing ununpentium isotopes directly. Data in bold represent maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 2n | 3n | 4n | 5n |
|---|---|---|---|---|---|---|
| 48Ca | 243Am | 291Uup | 3.7 pb, 39.0 MeV | 0.9 pb, 44.4 MeV |
Future experiments
The team at RIKEN are planning to study the reaction
As a primary next-goal for the Dubna team, they are planning to examine to products of the 243Am + 48Ca using mass spectrometry in their state-of-the-art MASHA machine. They will attempt to isolate the dubnium products, convert them chemically into a volatile compound, most likely 268DbCl5, and measure the mass directly.
See also
- Island of stability
- Elerium-115
- Bob Lazar
References
- ↑ "Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291−x115", Oganessian et al., Phys. Rev. C69, 021601 (2004). Retrieved on 2008-03-03
- ↑ "Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291−x115", Oganessian et al., JINR preprints (2003). Retrieved on 2008-03-03
- ↑ "RESULTS OF THE EXPERIMENT ON CHEMICAL IDENTIFICATION OF Db AS A DECAY PRODUCT OF ELEMENT 115", Oganessian et al., JINR preprints (2004). Retrieved on 2008-03-03
- ↑ "Synthesis of elements 115 and 113 in the reaction 243Am + 48Ca", Oganessian et al., Phys. Rev. C72, 034611 (2005). Retrieved on 2008-03-03
- ↑ C. Samanta, P. Roy Chowdhury and D.N. Basu (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A 789: 142–154. doi:. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TVB-4NF4F0Y-2&_user=2806701&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000058844&_version=1&_urlVersion=0&_userid=2806701&md5=3f680654b5659191d67f31681a4cfc83.
- ↑ Keller, O. L., Jr.; C. W. Nestor, Jr. (1974). "Predicted properties of the superheavy elements. III. Element 115, Eka-bismuth". Journal of Physical Chemistry 78: 1945. doi:.
- ↑ "List of experiments 2000-2006"
External links
- Uut and Uup Add Their Atomic Mass to Periodic Table
- Apsidium: Ununpentium
- Superheavy elements
- History and etymology
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||