Physical constant
A physical constant is a physical quantity that is generally believed to be both universal in nature and constant in time. It can be contrasted with a mathematical constant, which is a fixed numerical value but does not directly involve any physical measurement.
There are many physical constants in science, some of the most widely recognized being the rationalized Planck's constant h, the gravitational constant G, the speed of light in vacuum c, the electric constant ε0, and the elementary charge e. Physical constants can take many dimensional forms: the speed of light signifies a maximum speed limit of the universe and is expressed dimensionally as length divided by time; while the fine-structure constant α, which characterizes the strength of the electromagnetic interaction, is dimensionless.
Dimensionful and dimensionless physical constants
Whereas the values of physical constants do not depend on the unit system used, the numerical values of dimensionful physical constants do depend on the unit used. Therefore, these numerical values (such as 299,792,458 for the constant speed of light c expressed in units of meters per second) are not values that a theory of physics can be expected to predict.
Ratios of like-dimensioned physical constants do not depend on unit systems in this way (the units cancel), so they are pure (dimensionless) numbers whose values a future theory of physics could conceivably hope to predict. Additionally, all equations describing laws of physics can be expressed without dimensional physical constants via a process known as nondimensionalisation, but the dimensionless constants will remain. Thus, theoretical physicists tend to regard these dimensionless quantities as fundamental physical constants.
However, the phrase fundamental physical constant is also used in other ways. For example, the National Institute of Standards and Technology[1] uses it to refer to any universal physical quantity believed to be constant, such as the speed of light, c, and the gravitational constant G.
The fine-structure constant α is probably the best known dimensionless fundamental physical constant. Many attempts have been made to derive its value (currently measured at about 1/137.035999) from theory, but so far none have succeeded. The same holds for the dimensionless ratios of masses of fundamental particles (the most apparent is mp/me, approximately 1836.152673). With the development of quantum chemistry in the 20th century, however, a vast number of previously inexplicable dimensionless physical constants were successfully computed from theory. As such, some theoretical physicists still hope for continued progress in explaining the values of dimensionless physical constants.
It is known that the universe would be very different if these constants took values significantly different from those we observe. For example, a few percent change in the value of the fine structure constant would be enough to eliminate stars like our Sun. This has prompted attempts at anthropic explanations of the dimensionless physical constants.
How constant are the physical constants?
Beginning with Paul Dirac in 1937, some scientists have speculated that physical constants may actually decrease in proportion to the age of the universe. Scientific experiments have not yet pinpointed any definite evidence that this is the case, although they have placed upper bounds on the maximum possible relative change per year at very small amounts (roughly 10−5 per year for the fine structure constant α and 10−11 for the gravitational constant G).
It is currently disputed[2][3] that any changes in dimensional physical constants such as G, c, ħ, or ε0 are operationally meaningful;[4] however, a sufficient change in a dimensionless constant such as α is generally agreed to be something that would definitely be noticed. If a measurement indicated that a dimensional physical constant had changed, this would be the result or interpretation of a more fundamental dimensionless constant changing, which is the salient metric. From John D. Barrow 2002:
- "[An] important lesson we learn from the way that pure numbers like α define the world is what it really means for worlds to be different. The pure number we call the fine structure constant and denote by α is a combination of the electron charge, e, the speed of light, c, and Planck's constant, h. At first we might be tempted to think that a world in which the speed of light was slower would be a different world. But this would be a mistake. If c, h, and e were all changed so that the values they have in metric (or any other) units were different when we looked them up in our tables of physical constants, but the value of α remained the same, this new world would be observationally indistinguishable from our world. The only thing that counts in the definition of worlds are the values of the dimensionless constants of Nature. If all masses were doubled in value you cannot tell because all the pure numbers defined by the ratios of any pair of masses are unchanged."
Anthropic principle
Some physicists have explored the notion that if the (dimensionless) fundamental physical constants had sufficiently different values, our universe would be so radically different that intelligent life would probably not have emerged, and that our universe therefore seems to be fine-tuned for intelligent life. The Strong anthropic principle states that it must be because these fundamental constants acquired their respective values that there was sufficient order in the Universe and richness in elemental diversity for life to have formed, which subsequently evolved the necessary intelligence toward observing that these constants have taken on the values they have, which then allowed for our privileged perspective from the Weak anthropic principle standpoint.
Table of universal constants
| Quantity | Symbol | Value | Relative Standard Uncertainty |
|---|---|---|---|
| speed of light in vacuum |  |
299 792 458 m·s−1 | defined |
| Newtonian constant of gravitation | 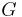 |
6.67428(67) × 10−11m³·kg−1·s−2 | 1.0 × 10−4 |
| Planck's constant | 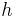 |
6.626 068 96(33) × 10−34 J·s | 5.0 × 10−8 |
| Dirac's constant | 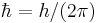 |
1.054 571 628(53) × 10−34 J·s | 5.0 × 10−8 |
Table of electromagnetic constants
| Quantity | Symbol | Value[5] (SI units) | Relative Standard Uncertainty |
|---|---|---|---|
| magnetic constant (vacuum permeability) | 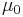 |
4π × 10−7 N·A−2 = 1.256 637 061... × 10−6 N·A−2 | defined |
| electric constant (vacuum permittivity) | 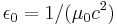 |
8.854 187 817... × 10−12 F·m−1 | defined |
| characteristic impedance of vacuum | 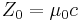 |
376.730 313 461... Ω | defined |
| Coulomb's constant | 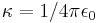 |
8.987 551 787 4 × 109 N·m²·C−2 | defined |
| elementary charge | 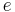 |
1.602 176 487(40) × 10−19 C | 2.5 × 10−8 |
| Bohr magneton | 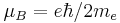 |
927.400 915(23) × 10−26 J·T−1 | 2.5 × 10−8 |
| conductance quantum | 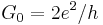 |
7.748 091 7004(53) × 10−5 S | 6.8 × 10−10 |
| inverse conductance quantum | 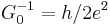 |
12 906.403 7787(88) Ω | 6.8 × 10−10 |
| Josephson constant | 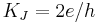 |
483 597.891(12) × 109 Hz·V−1 | 2.5 × 10−8 |
| magnetic flux quantum | 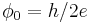 |
2.067 833 667(52) × 10−15 Wb | 2.5 × 10−8 |
| nuclear magneton | 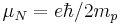 |
5.050 783 43(43) × 10−27 J·T−1 | 8.6 × 10−8 |
| von Klitzing constant | 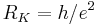 |
25 812.807 557(18) Ω | 6.8 × 10−10 |
Table of atomic and nuclear constants
| Quantity | Symbol | Value[5] (SI units) | Relative Standard Uncertainty | |
|---|---|---|---|---|
| Bohr radius | 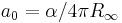 |
0.529 177 2108(18) × 10−10 m | 3.3 × 10−9 | |
| classical electron radius | 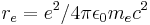 |
2.817 940 2894(58) × 10−15 m | 2.1 × 10−9 | |
| electron mass | 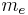 |
9.109 382 15(45) × 10−31 kg | 5.0 × 10−8 | |
| Fermi coupling constant | 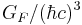 |
1.166 39(1) × 10−5 GeV−2 | 8.6 × 10−6 | |
| fine-structure constant | 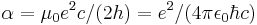 |
7.297 352 568(24) × 10−3 | 3.3 × 10−9 | |
| Hartree energy | 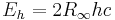 |
4.359 744 17(75) × 10−18 J | 1.7 × 10−7 | |
| proton mass | 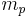 |
1.672 621 637(83) × 10−27 kg | 5.0 × 10−8 | |
| quantum of circulation | 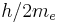 |
3.636 947 550(24) × 10−4 m² s−1 | 6.7 × 10−9 | |
| Rydberg constant | 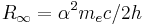 |
10 973 731.568 525(73) m−1 | 6.6 × 10−12 | |
| Thomson cross section | 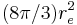 |
0.665 245 873(13) × 10−28 m² | 2.0 × 10−8 | |
| weak mixing angle | 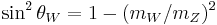 |
0.222 15(76) | 3.4 × 10−3 | |
Table of physico-chemical constants
| Quantity | Symbol | Value[5] (SI units) | Relative Standard Uncertainty | |
|---|---|---|---|---|
| atomic mass unit (unified atomic mass unit) | 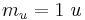 |
1.660 538 86(28) × 10−27 kg | 1.7 × 10−7 | |
| Avogadro's number | 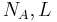 |
6.022 1415(10) × 1023 mol−1 | 1.7 × 10−7 | |
| Boltzmann constant | 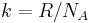 |
1.380 6505(24) × 10−23 J·K−1 | 1.8 × 10−6 | |
| Faraday constant | 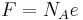 |
96 485.3383(83)C·mol−1 | 8.6 × 10−8 | |
| first radiation constant | 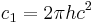 |
3.741 771 18(19) × 10−16 W·m² | 5.0 × 10−8 | |
| for spectral radiance | 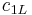 |
1.191 042 82(20) × 10−16 W·m² sr−1 | 1.7 × 10−7 | |
| Loschmidt constant | at  =273.15 K and =273.15 K and 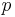 =101.325 kPa =101.325 kPa |
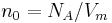 |
2.686 7773(47) × 1025 m−3 | 1.8 × 10−6 |
| gas constant | 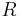 |
8.314 472(15) J·K−1·mol−1 | 1.7 × 10−6 | |
| molar Planck constant | 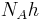 |
3.990 312 716(27) × 10−10 J·s·mol−1 | 6.7 × 10−9 | |
| molar volume of an ideal gas | at  =273.15 K and =273.15 K and 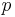 =100 kPa =100 kPa |
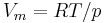 |
22.710 981(40) × 10−3 m³·mol−1 | 1.7 × 10−6 |
at  =273.15 K and =273.15 K and 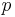 =101.325 kPa =101.325 kPa |
22.413 996(39) × 10−3 m³·mol−1 | 1.7 × 10−6 | ||
| Sackur-Tetrode constant | at  =1 K and =1 K and 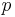 =100 kPa =100 kPa |
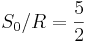 ![+ \ln\left[ (2\pi m_u k T / h^2)^{3/2} k T / p \right]](/2009-wikipedia_en_wp1-0.7_2009-05/I/358b3e034bd9f1c891bbb05233461a70.png) |
−1.151 7047(44) | 3.8 × 10−6 |
at  =1 K and =1 K and 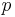 =101.325 kPa =101.325 kPa |
−1.164 8677(44) | 3.8 × 10−6 | ||
| second radiation constant | 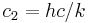 |
1.438 7752(25) × 10−2 m·K | 1.7 × 10−6 | |
| Stefan-Boltzmann constant | 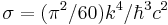 |
5.670 400(40) × 10−8 W·m−2·K−4 | 7.0 × 10−6 | |
| Wien displacement law constant | 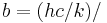 4.965 114 231... 4.965 114 231... |
2.897 7685(51) × 10−3 m·K | 1.7 × 10−6 | |
Table of adopted values
| Quantity | Symbol | Value (SI units) | Relative Standard Uncertainty | |
|---|---|---|---|---|
| conventional value of Josephson constant[6] | 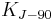 |
483 597.9 × 109 Hz·V−1 | defined | |
| conventional value of von Klitzing constant[7] | 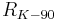 |
25 812.807 Ω | defined | |
| molar mass | constant | 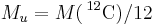 |
1 × 10−3 kg·mol−1 | defined |
| of carbon-12 | 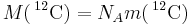 |
12 × 10−3 kg·mol−1 | defined | |
| standard acceleration of gravity (gee, free fall on Earth) | 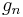 |
9.806 65 m·s−2 | defined | |
| standard atmosphere | 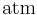 |
101 325 Pa | defined | |
Notes
- ↑ Latest (2006) Values of the Constants; NIST, 2006.
- ↑ Duff, Michael J. "Comment on time-variation of fundamental constants." High Energy Physics - Theory, 2004.
- ↑ Duff, M. J.; Okun, L. B.; Veneziano, G. "Trialogue on the number of fundamental constants." Classical Physics, 2002.
- ↑ c, and ε0 now are defined numerical values, independent of experiment, so observations now are trained elsewhere, for example, upon a changing value of the meter.
- ↑ 5.0 5.1 5.2 The values are given in the so-called concise form; the number in brackets is the standard uncertainty, which is the value multiplied by the relative standard uncertainty.
- ↑ This is the value adopted internationally for realizing representations of the volt using the Josephson effect.
- ↑ This is the value adopted internationally for realizing representations of the ohm using the quantum Hall effect.
References
- CODATA Recommendations - 2006 CODATA Internationally recommended values of the Fundamental Physical Constants
- Barrow, John D., The Constants of Nature; From Alpha to Omega - The Numbers that Encode the Deepest Secrets of the Universe. Pantheon Books, 2002. ISBN 0-375-42221-8.
- Mohr, Peter J., Taylor, Barry N., Newell, David B., CODATA Recommended Values of the Fundamental Physical Constants: 2006