Trinitrotoluene
| Trinitrotoluene | |
|---|---|
 |
 |
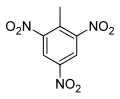
| IUPAC name | 2-methyl-1,3,5-trinitrobenzene |
| Other names | 2,4,6-Trinitrotoluene, TNT, Trilite, Tolite, Trinol, Trotyl, Tritolo, Tritolol, Triton, Tritone, Trotol, Trinitrotoluol, 2,4,6-Trinitromethylbenzene |
| Identifiers | |
| Abbreviations | TNT |
| CAS number | 118-96-7 |
| PubChem | |
| UN number | 0209 – Dry or wetted with < 30% water 0388, 0389 – Mixtures with trinitrobenzene, hexanitrostilbene |
| SMILES |
|
| Properties | |
| Molecular formula | C7H5N3O6 |
| Molar mass | 227.131 g/mol |
| Appearance | Pale yellow. Loose "needles" before melt-casting. A solid block after being poured into a casing. |
| Density | 1.654 g/cm³ |
| Melting point |
80.35 °C |
| Boiling point |
295 °C (decomposition) |
| Solubility in water | 130 mg/L of H2O (20 °C) |
| Solubility | ether acetone benzene pyridine |
| Explosive data | |
| Shock sensitivity | Insensitive |
| Friction sensitivity | Insensitive to 353 N |
| Explosive velocity | 6,900 m/s |
| RE factor | 1.00 |
| Hazards | |
| NFPA 704 |
 4
3
4
|
| R-phrases | R2 R23/24/25 R33 R51/53 |
| S-phrases | S35 S45 S61 |
| Related compounds | |
| Related compounds | picric acid hexanitrobenzene |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Trinitrotoluene (TNT) is a chemical compound with the formula C6H2(NO2)3CH3. This yellow-coloured solid is a reagent (reactant) in chemistry but is best known as a useful explosive material with convenient handling properties. The explosive yield of TNT is considered the standard measure of strength of bombs and other explosives. In chemistry, TNT is used to generate charge transfer salts.
Contents |
Preparation
TNT is synthesized in a three-step process.[1] First, toluene is nitrated with a mixture of sulfuric and nitric acid to produce mono-nitrotoluene or MNT. The MNT is then nitrated to dinitrotoluene or DNT. In the final step, the DNT is nitrated to trinitrotoluene or TNT. The acids used in the manufacture of TNT are recycled and reused.
Applications
TNT is one of the most commonly used explosives for military and industrial applications. It is valued because of its insensitivity to shock and friction, which reduces the risk of accidental detonation. TNT melts at 80 °C (176 °F), far below the temperature at which it will spontaneously detonate, allowing it to be poured as well as safely combined with other explosives. TNT neither absorbs nor dissolves in water, which allows it to be used effectively in wet environments. Additionally, it is relatively stable when compared to other high explosives.
Although blocks of TNT are available in various sizes (eg 250 g, 500 g, 1,000 g and even 20 kg[2]), it is more commonly encountered in synergistic explosive blends comprising a variable percentage of TNT plus other ingredients. Examples of explosive blends containing TNT include:
- Amatol
- Ammonal
- Baratol
- Composition B
- Composition H6
- Ednatol
- Hexanite
- Minol
- Octol
- Pentolite
- Picratol
- Tetrytol
- Torpex
- Tritonal
Explosive character
It is a common misconception that TNT and dynamite are the same, or that dynamite contains TNT. In fact, whereas TNT is a specific chemical compound, dynamite is an absorbent mixture soaked in nitroglycerin that is compressed into a cylindrical shape and wrapped in paper.
Upon detonation, TNT decomposes as follows:
- 2 C7H5N3O6 → 3 N2 + 5 H2O + 7 CO + 7 C
The reaction is exothermic but has a high activation energy. Because of the production of carbon, TNT explosions have a sooty appearance.
For many years, TNT used to be the reference point for the Figure of Insensitivity. TNT has a rating of exactly 100 on the F of I scale. However, the reference has since been changed to a more sensitive explosive called RDX, which has an F of I of 80.
History
TNT was first prepared in 1863 by German chemist Joseph Wilbrand[3] and originally used as a yellow dye. Its potential as an explosive was not appreciated for several years mainly because it was so difficult to detonate and because it was less powerful than alternatives. TNT can be safely poured when liquid into shell cases, and is so insensitive that in 1910, it was exempted from the UK's Explosives Act 1875 and was not considered an explosive for the purposes of manufacture and storage.
The German armed forces adopted it as a filling for artillery shells in 1902. TNT-filled armour-piercing shells would explode after they had penetrated the armour of British capital ships, whereas the British lyddite-filled shells tended to explode upon striking armour, thus expending much of their energy outside the ship. The British started replacing lyddite with TNT in 1907. TNT is still widely used by the United States military and construction companies around the world. The majority of TNT currently used by the US military is manufactured by Radford Army Ammunition Plant near Radford, Virginia.

Safety and toxicity
TNT is poisonous, and skin contact can cause skin irritation, causing the skin to turn a bright yellow-orange color. During the First World War, munition workers who handled the chemical found that their skin turned bright yellow, which resulted in their acquiring the nickname "canary girls" or simply "canaries." People exposed to TNT over a prolonged period tend to experience anemia and abnormal liver functions. Blood and liver effects, spleen enlargement and other harmful effects on the immune system have also been found in animals that ingested or breathed trinitrotoluene. There is evidence that TNT adversely affects male fertility, and TNT is listed as a possible human carcinogen[4]. Consumption of TNT produces red urine through the presence of breakdown products and not blood as sometimes believed.[5]
Some military testing grounds are contaminated with TNT. Wastewater from munitions programs including contamination of surface and subsurface waters may be colored pink because of the presence of TNT. Such contamination, called "pink water", may be difficult and expensive to remedy.
See also
- Megaton
- Explosives used during WW II
References
- ↑ Army TM 9-1300-214, p. 8-83
- ↑ Industria de Material Belico do Brasil
- ↑ J. Wilbrand (1863). "Notiz über Trinitrotoluol". Annalen der Chemie und Pharmacie 128 (2): 178–179. doi:.
- ↑ Toxicological Profile for 2,4,6-Trinitrotoluene
- ↑ "2,4,6-Trinitrotoluene". Agency for Toxic Substances and Disease Registry (September 1996). Retrieved on 2008-02-02.
External links
- Video showing detonation of 50 tons of TNT in 1963 during Anglo-Australian Operation Blowdown
- Video showing the five 20 ton underground blasts of Project Dugout in 1965
- Video showing the shockwave and typical black smoke cloud from detonation of 160 kilograms of pure TNT
- Video showing detonation of 453 metric tons of TNT in 1965 Operation Sailor Hat - note shockwave and black smoke residue
- Video of demolition training using half pound blocks of pure TNT
- Arc Blast TNT Equivalent Calculator