Trilobite
| Trilobites Fossil range: 530–251.4 Ma Early Cambrian - Late Permian |
||||||
|---|---|---|---|---|---|---|
 Asaphus kowalewskii
|
||||||
| Scientific classification | ||||||
|
||||||
| Orders | ||||||
Subclass: Librostoma
|
Trilobites ("three-lobes") are extinct arthropods that form the class Trilobita. They appeared in the Early Cambrian period and flourished throughout the lower Paleozoic era before beginning a drawn-out decline to extinction when, during the Late Devonian extinction, all trilobite orders, with the sole exception of Proetida, died out. The last of the trilobites disappeared in the mass extinction at the end of the Permian about 250 million years ago (m.y.a.).
Trilobites are very well-known, and possibly the second-most famous fossil group, after the dinosaurs. When trilobites appear in the fossil record of the Lower Cambrian they were already highly diverse and geographically dispersed. Because of their diversity and an easily fossilized exoskeleton, they left an extensive fossil record with some 17,000 known species spanning Paleozoic time. Trilobites have been important in biostratigraphy, paleontology, and plate tectonics research. Trilobites are often placed within the arthropod subphylum Schizoramia within the superclass Arachnomorpha (equivalent to the Arachnata),[1] although several alternative taxonomies are found in the literature.
Different trilobites made their living in different ways. Some led a benthic life as predators, scavengers or filter feeders. Some swam (a pelagic lifestyle) and fed on plankton. Most life styles expected of modern marine arthropods are seen, except for parasitism.[2] Some trilobites (particularly the family Olenida) are even thought to have evolved a symbiotic relationship with sulfur-eating bacteria from which they derived food.[3]
Contents |
Phylogeny
Despite their rich fossil record with thousands of genera found throughout the world, the taxonomy and phylogeny of trilobites have many uncertainties. The systematic division of trilobites into nine distinct orders is represented by a widely held view that will inevitably change as new data emerge. Except possibly for the members of order Phacopida, all trilobite orders appeared prior to the end of the Cambrian. Most scientists believe that order Redlichiida, and more specifically its suborder Redlichiina, contains a common ancestor of all other orders, with the possible exception of the Agnostina. While many potential phylogenies are found in the literature, most have suborder Redlichiina giving rise to orders Corynexochida and Ptychopariida during the Lower Cambrian, and the Lichida descending from either the Redlichiida or Corynexochida in the Middle Cambrian. Order Ptychopariida is the most problematic order for trilobite classification. In the 1959 Treatise on Invertebrate Paleontology,[4] what are now members of orders Ptychopariida, Asaphida, Proetida, and Harpetida were grouped together as order Ptychopariida; subclass Librostoma was erected in 1990[5] to encompass all of these orders, based on their shared ancestral character of a natant (unattached) hypostome. The most recently recognized of the nine trilobite orders, Harpetida, was erected in 2002.[6] The progenitor of order Phacopida is unclear.
Terminology
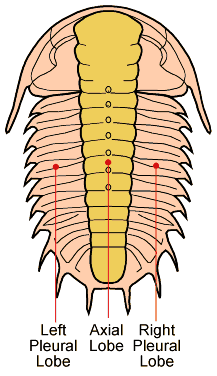
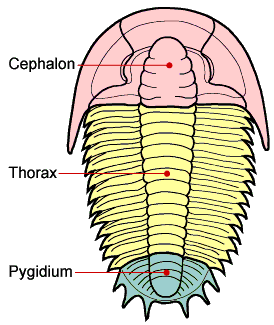
As might be expected for a group of animals comprising 1,500+ genera and 17,000+ species the morphology and description of trilobites can be complex. "Trilobite" (meaning "three-lobed") is named for the three longitudinal lobes: a central axial lobe, and two symmetrical pleural lobes that flank the axis (Fig 1). The trilobite body is divided into three major sections, a cephalon (head) with eyes, mouth parts and antennae, a thorax of multiple similar segments (that in some species allowed enrollment), and a pygidium (tail), see Fig 2. When describing differences between different taxa of trilobites, the presence, size, and shape of the cephalic features are often mentioned and shown in Figs 3 & 4.
Physical description
When trilobites are found, only the exoskeleton is preserved (often in an incomplete state) in all but a handful of locations. A few locations (Lagerstätten) preserve identifiable soft body parts (legs, gills, musculature & digestive tract) and enigmatic traces of other structures (e.g. fine details of eye structure) as well as the exoskeleton.
Trilobites range in length from 1 mm to 72 cm (1/25 inch to 28 inches), with a typical size range of 3 to 10 cm (1 to 4 inches). The world's largest trilobite, Isotelus rex, was found in 1998 by Canadian scientists in Ordovician rocks on the shores of Hudson Bay.[7]
Exoskeleton
The exoskeleton is composed of calcite and calcium phosphate minerals in a protein lattice of chitin that covers the upper surface (dorsal) of the trilobite and curled round to produce a small fringe beneath called the doublure. The exoskeleton of trilobites are divided into three parts (tagmata): a cephalon, composed of the two preoral and first four postoral segments completely fused together; a thorax composed of freely articulating segments; and a pygidium composed of the last segments fused together with the telson.
During molting, the exoskeleton generally split between the head and thorax, which is why so many trilobite fossils are missing one or the other. In most groups there were facial sutures on the cephalon to facilitate molting.
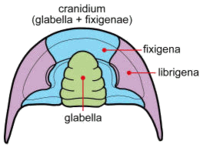
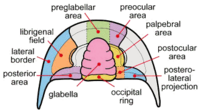
Cephalon
The cephalon of trilobites is highly variable with a lot of morphological complexity. The glabella forms a dome underneath which sat the "crop" or "stomach". The ventral surface of the cephalon often preserves muscle attachment scars and the presence of the hypostome, a small rigid plate upon which sat the mouth and stomach. The hypostome is highly variable, sometimes supported by a membrane (natant), sometimes fused onto the doublure with an outline very similar to the glabella above (conterminant) or fused to the doublure with an outline significantly different from the glabella (impendent). Many variations in shape and placement of the hyperstome have been described.[5] Size of the glabella, lateral fringe and hypostome variation have all been linked to different lifestyles, diets and ecological niches.[2] The fringe of the cephalon is greatly exaggerated in the Harpetida, in other species a bulge in the pre-glabellar area is preserved that suggests a brood pouch.[8] Highly complex compound eyes are another obvious feature of the cephalon (see below). Figure 3 shows gross morphology of the cephalon. The cheeks (genae) are the pleural lobes on each side of the axial feature, the glabella. When trilobites molted or died, the librigenae (the so-called "free cheeks") often separated, leaving the cranidium (glabella + fixigenae) exposed. Figure 4 shows a more detailed view of the cephalon.
Thorax
The thorax is s series of articulated segments that lie between the cephalon and pygidium. Number of segments varies between 2 and 61 with most species in the 2 to 16 range.[9] Each segment consists of the central axial ring and the outer plurae which protected the limbs and gills. The plurae are sometimes abbreviated to save weight or extended to form long spines. Apodemes are bulbous projections on the underside to which most leg muscles attached, athough some leg muscles attached directly to the exoskeleton.[10] Distinguishing where the thorax ends and the pygidium begins can be problematic and many segment counts suffer from this problem.[9]

Fossilised trilobites are often found enrolled (curled up) like modern woodlice for protection, evidence suggests enrollment helped protect against exploitation of arthropod cuticle weakness by Anomalacarid predator attacks.[11] Some trilobites achieved a fully closed capsule (e.g. Phacops) while others with long pleural spines (e.g. Selenopeltis) or a small pygidium (e.g. Paradoxides) left a gap at the sides or between the cephalon and pygidium .[9] Even in an Agnostid, with only 2 articulating thoracic segments, the process of enrollment required a complex musculature to contract the exoskeleton and return to the flat condition.[12] In Phacops the pleurae overlap a smooth bevel (facet) allowing a close seal with the doublure. The doublure carries a panderian notch or protuberance on each segment to prevent over rotation of each segment. Long lateral muscles extended from the cephalon to mid way down the pygidium, attaching to the axial rings allowing enrollment while separate muscles on the legs tucked them out of the way.[10]
Pygidium
Is a number of segments and the telson fused together to form the tail. The pygidium segments are similar to the thoracic segments bearing legs and gills. Trilobites can be described based on the pydigium being micropygous (pydigium smaller than cephalon), isopygous (pydigium equal in size to cephalon), or macropygous (pydigium larger than cephalon).
Prosopon (surface sculpture)
Trilobite exoskeletons show a variety of small-scale structures collectively called prosopon. Prosopon does not include large scale extensions of the cuticle (e.g. hollow pleural spines) but to finer scale features, such as ribbing, domes, pustules, pitting, ridging and perforations. The exact purpose of the prosopon is not resolved but suggestions include structural strengthening, sensory pits or hairs, preventing predator attacks and maintaining aeration while enrolled.[9] In one example, alimentary ridge networks (easily visible in Cambrian trilobites) might have been either digestive or respiratory tubes in the cephalon and other regions.[13] Later, more advanced trilobites developed thicker cuticles (making alimentary prosopon harder to see) against predation by cephalopods.
Spines
Some trilobites such as those of the order Lichida evolved elaborate spiny forms, from the Ordovician until the end of the Devonian period. Examples of these specimens have been found in the Hamar Laghdad Formation of Alnif in Morocco. Collectors of this material should be aware of a serious counterfeiting and fakery problem with much of the Moroccan material that is offered commercially. Spectacular spined trilobites have also been found in western Russia; Oklahoma, USA; and Ontario, Canada. These spiny forms could possibly have been a defensive response to the evolutionary appearance of fish.
Some trilobites had horns on their heads similar to those of modern beetles. Based on the size, location, and shape of the horns the most likely use of the horns was combat for mates, making the Asaphida family Raphiophoridae the earliest exemplars of this behavior.[14] A conclusion likely to be applicable to other trilobites as well, such as in the Phacopid trilobite genus Walliserops that developed spectacular tridents.[15]

Soft Body Parts
Legs & Gills
Trilobites had a single pair of preoral antennae and otherwise undifferentiated biramous limbs. Each exopodite (walking leg) had six segments, homologous to other early arthropods. The first segment also bore a feather-like epipodite, or gill branch, which was used for respiration and, in some species, swimming. The last expodite segment had a claw and 2 articulated flanking hooks.[10] Many examples of hairs on the legs suggest adaptations for feeding or sensory organs to help with walking.[16]
Digestive tract
The mouth of trilobites was situated on the rear edge of the hypostome, in front of the 2 pairs of legs attached to the cephalon. The mouth is linked by a small oesophagus to the stomach that lay forward of the mouth, below the glabella. The "intestine" led backwards from there to the pygidium.[10]
Sensory organs
Many trilobites had eyes; they also had antennae that perhaps were used for taste and smell. Some trilobites were blind, probably living too deep in the sea for light to reach them. As such, they became secondarily blind in this branch of trilobite evolution. Others, such as Phacops rana, had eyes that were quite large for use in more well lit, predator-filled waters.
Antennae
The pair of antennae suspected in most trilobites (but preserved only in a few) were highly flexible to allow them to be retracted when the trilobite was enrolled. The antennae are probably similar to those in extant arthropods and as such could sense touch, water motion, heat, vibration (sound), and especially olfaction (smell) or gustation (taste).
Eyes
Even the earliest trilobites had complex, compound eyes with lenses made of calcite, a unique characteristic of all trilobite eyes. This confirms that eyes of arthropods and probably other animals were already quite developed at the beginning of the Cambrian. Improving eyesight of both predator and prey in marine environments probably provided one of the evolutionary pressures furthering an apparent rapid development of new life forms during what is known as the Cambrian Explosion.
The trilobite eyes were typically compound, with each lens being an elongated prism. The number of lenses in such an eye varied: some trilobites had only one, while some had thousands of lenses in a single eye. In these compound eyes, the lenses were typically arranged hexagonally.[13] The fossil record of trilobite eyes is complete enough that their evolution can be studied through time, which compensates to some extent the lack of preservation of soft internal parts.[17]
The lenses of trilobites eyes were made of calcite (calcium carbonate, CaCO3). Pure forms of calcite are transparent, and some trilobites used crystallographically oriented, clear calcite crystals to form each lens of each of their eyes.[18] In this, they differ from most other arthropods, which have soft or chitin-supported eyes.
The rigid calcite lenses of a trilobite eye would have been unable to accommodate to a change of focus like the soft lens in a human eye would; however, in some trilobites the calcite formed an internal doublet structure,[19] giving superb depth of field and minimal spherical aberration, as rediscovered by French scientist René Descartes and Dutch physicist Christiaan Huygens many millions of years later. A living species with similar lenses is the brittle star Ophiocoma wendtii. In other trilobites, with a Huygens interface apparently missing, a gradient index lens is invoked with the refractive index of the lens changing towards the center.[20]
Holochroal eyes had a great number (sometimes over 15,000) of small (30-100μm, rarely larger)[17] lenses. Lenses were hexagonally close packed, touching each other, with a single corneal membrane covering all lenses.[18] Holochroal eyes had no sclera, the white layer covering the eyes of most modern arthropods. Holochroal eyes are the ancestral eye of trilobites, and are by far the most common, found in all orders and through the entirety of the Trilobites' existance.[17] Little is known of the early history of holochroal eyes; Lower and Middle Cambrian trilobites rarely preserve the visual surface.[17]
Schizochroal eyes typically had fewer (to around 700), larger lenses than holochroal eyes and are found only in Phacopida. Lenses were separate, with each lens having an individual cornea which extended into a rather large sclera.[18] Schizochroal eyes appear quite suddenly in the early Ordovician, and were presumably derived from a holochroal ancestor.[17] Field of view (all around vision), eye placement and coincidental development of more efficient enrollment mechanisms point to the eye as a more defensive "early warning" system than directly aiding in the hunt for food.[17] Eyes which are functionally equivalent to the schizochroal eye are found in the modern insect species Xenos peckii.[22]
Abathochroal eyes had around 70 small lenses, and are found only in Cambrian Eodiscina. Each lens was separate and had an individual cornea. The sclera was separate from the cornea, and did not run as deep as the sclera in schizochroal eyes.[18]
Secondary blindness is not uncommon, particularly in long lived groups such as the Agnostida and Trinucleioidea. In Proetida, Phacopina and Tropidocoryphinae there are well studied trends showing progressive eye reduction between closely related species that eventually leads to blindness.[18]
Several other structures on trilobites have been explained as photo-receptors.[18] Of particular interest are the small areas of thinned cuticle on the underside of the hypostome (macula) which, in some trilobites, are suggested to be simple ventral eyes that could have detected night and day or allowed a trilobite to navigate while swimming (or turned) upside down.[20]
Sensory Pits
There are several types of prosopon that have been suggested as sensory apparatus collecting chemical or vibrational signals. The connection between large pitted fringes on the cephalon of Harpetida and Trinucleoidea with corresponding small or absent eyes makes for an interesting possibility of the fringe as a "compound ear".[18]
Development
Trilobites grew through successive molt stages called instars, in which existing segments increased in size and new trunk segments appeared at a sub-terminal generative zone during the "anamorphic" phase of development. The molt itself, is called ecdysis. This was followed by the "epimorphic" developmental phase, in which the animal continued to grow and molt, but no new trunk segments were expressed in the exoskeleton. The combination of anamorphic and epimorphic growth consistutes the "hemianamorphic" developmental mode that is common among many living arthropods.
Trilobite development was unusual in the way in which articulations developed between segments, and changes in the development of articulation gave rise to the conventionally recognized developmental phases of the trilobite life cycle (divided into 3 stages), which are not readily compared with those of other arthropods. Actual growth and change in external form of the trilobite would have occurred when the trilobite was soft shelled, following molting and before the next hard exoskeleton.[23]
Trilobite larvae are known from the Cambrian to the Carboniferous[24] and from all sub-orders.[23][25] As instars from closely related taxa are more similar than instars from distantly related taxa, trilobite larvae provide morphological information important in evaluating high-level phylogenetic relationships among trilobites.[23]
Trilobites are thought to have reproduced sexually, producing eggs, albeit without undoubted examples in the fossil record.[23] Some species may have kept eggs or larvae in a brood pouch forward of the glabella,[8] particularly when the ecological niche was particularly challenging to larvae.[3] Size and morphology of the first calcified stage are highly variable between (but not within) trilobite taxa, suggesting some trilobites passed through more growth within the egg than others. Early developmental stages prior to calcification of the exoskeleton are a possibility, but so is calcification and hatching coinciding.[23]
The earliest post-embryonic trilobite growth stage known with certainty are the protaspid stages.[23] Starting with an indistinguishable proto-cephalon and proto-pygidium (anaprotaspid) a number of changes occur ending with a transverse furrow separating the proto-cephalon and proto-pygidium (metaprotaspid) that can continue to add segments. Segments are added at the posterior part of the pygidium but, all segments remain fused together.[23][25]
The meraspid phase of development is marked by the appearance of an articulation between the head and the fused trunk. At the onset of the meraspid phase the animal had a two-part structure - the head and the plate of fused trunk segments, the pygidium. During the meraspid phase, new segments appeared near the rear of the pygidium as additional articulations developed at the anterior of the pygidium, releasing freely articulating thoracic segments. Segments are generally added one per molt (although two per molt and one every alternate molt are also recorded), with number of stages equal to the number of thoracic segments. A substantial amount of growth, from less than 25% up to 30-40%, probably took place in the meraspid stages.[23]
The holaspid phase of growth commenced when a stable, mature number of segments had been released into the thorax. Molting continued during the holaspid stage, with no changes in thoracic segment number.[23] Onset of the holaspid phase and the epimorphic phase was coincident in some, but not all, trilobites.
Some trilobites showed a marked transition in morphology at one particular instar, which has been called trilobite metamorphosis. Radical change in morphology is linked to the loss or gain of distinctive features that mark a change in mode of life.[26] A change in lifestyle during development has significance in terms of evolutionary pressure, as the trilobite could pass through several ecological niches on the way to adult development and changes would strongly affect survivor-ship and dispersal of trilobite taxa.[23] It is worth noting that trilobites with all protaspid stages planktonic and meraspid stages benthic (e.g. Asaphids) failed to last through the Ordovician extinctions, while trilobites that were planktonic for only the first protaspid stage before metamorphosing into benthic forms survived (e.g. Lichids, Phacopids).[26]. Pelagic larval life-style proved ill-adapted to the rapid onset of global climatic cooling and loss of tropical shelf habitats during the Ordovician.[7]
Fossil record
The earliest trilobite known from the fossil record is the genus Fallotaspis within Order Redlichiida, dated to some .[27] Other early genera include Profalloptaspis and Eofallotaspis, all appearing about the same time.
Origins
Based on morphological similarities, it is possible that the trilobites have their ancestors in arthropod-like creatures such as Spriggina, Parvancorina, and other trilobitomorphs of the Ediacaran period of the Precambrian. There are many morphological similarities between early trilobites and other Cambrian arthropods known from the Burgess Shale, the Maotianshan shales at Chengjiang and other fossiliferous locations. These are investigated further here: [1] It is reasonable to assume that the trilobites share a common ancestor with these other arthropods prior to the Ediacaran-Cambrian boundary. Ancestral trilobites may have been somewhat soft bodied and developed their thick carapaces through Cuticularisation. As with other forms of trilobite body evolution, this was a defensive measure.
Extinction
The reason for the extinction of the trilobites is not clear, although it may be no coincidence that their numbers began to decrease with the appearance of the first sharks and other early gnathostomes in the Silurian and their subsequent rise in diversity during the Devonian period. Trilobites may have provided a rich source of food for these new animals. A smaller extinction event in the Middle Cambrian of trilobite orders possessing alimentary prosopon and a micropygidium may have been linked to the rise of cephalopods. Trilobites were under great selective pressure to develop defensive bodies quickly. The most radical change in body form occurred in the Middle Cambrian. As a means of defense, surviving orders developed isopygidius or macropygius bodies. This enabled trilobites to curl their bodies into a ball as a means of defense. A micropygidius trilobite cannot completely protect itself in a curled position with a pygidium smaller than the cephalon. It is analogous to pleurodirian (side-necked) turtles of the present day (Holocene). A terrestrial side neck could never evolve because the exposed neck in a side withdraw state would be vulnerable to a predator. Surviving trilobites developed thicker cuticles (as mentioned earlier) and as such, the alimentary prosopon are no longer visible due to the thickness.
After the mid-Cambrian extinction event, the next great extinction event occurred at the Frasnian - Famennian boundary at the end of the Devonian period. All orders (except one) of Trilobites became extinct. Trilobites were bottlenecked into one single order, the Proetida. This single order survived for millions of years, continued through the Carboniferous period and lasted to the great extinction event at the end of the Permian (where the vast majority of species on earth were wiped out). It is unknown why Order Proedita alone, survived. It may have been a deeper water order that was able to avoid rapid changes that would affect species along the continental shelves. For many millions of years, the Proetida found a perfect niche. An anology would be today's crinoids which exist as deep water species only. In the Paleozoic era, vast 'forests' of crinoids lived in shallow near shore environments.
Additionally, their relatively low numbers and diversity at the end of the Permian no doubt contributed to their extinction during that great mass extinction event. Foreshadowing this, the Ordovician mass extinction, though somewhat less substantial than the Permian one, also seems to have significantly narrowed trilobite diversity.
The closest extant relatives of trilobites may be the horseshoe crabs,[27] or the cephalocarids.[28]
Fossil distribution

Trilobites appear to have been exclusively marine organisms, since the fossilized remains of trilobites are always found in rocks containing fossils of other salt-water animals such as brachiopods, crinoids, and corals. Within the marine paleoenvironment, trilobites were found in a broad range from extremely shallow water to very deep water. Trilobites, like brachiopods, crinoids, and corals, are found on all modern continents, and occupied every ancient ocean from which Paleozoic fossils have been collected. The remnants of trilobites can range from the preserved body to pieces of the exoskeleton, which it sheds in the process known as ecdysis. In addition, the tracks left behind by trilobites living on the sea floor are often preserved as trace fossils.
There are three main forms of trace fossils associated with trilobites: Rusophycus; Cruziana & Diplicnites. These trace fossils represent the preserved life activity of trilobites active upon the sea floor. Rusophycus, the resting trace, are trilobite excavations which involve little or no foreward movement and ehtological interpretations suggest resting, protection and hunting. [29] Cruziana, the feeding trace, are furrows through the sediment, which are believed to represent the movement of trilobites while deposit feeding.[30] Diplichnites, the walking trace, are traces made by trilobites walking on the sediment surface.[30] These same trace fossils are also occasionally found in freshwater environments,[31] suggesting either that some freshwater trilobites existed, or that the tracks are also made by other organisms.
Trilobite fossils are found worldwide, with many thousands of known species. Because they appeared quickly in geological time, and moulted like other arthropods, trilobites serve as excellent index fossils, enabling geologists to date the age of the rocks in which they are found. They were among the first fossils to attract widespread attention, and new species are being discovered every year. Some Native Americans, recognizing that trilobites were water creatures, had a name for them which means "little water bug in the rocks".

A famous location for trilobite fossils in the United Kingdom is Wren's Nest, Dudley in the West Midlands, where Calymene blumenbachi is found in the Silurian Wenlock Group. This trilobite is featured on the town's coat of arms and was named the Dudley Bug or Dudley Locust by quarrymen who once worked the now abandoned limestone quarries. Other trilobites found there include Dalmanites, Trimerus, Bumastus and Balizoma. Llandrindod Wells, Powys, Wales, is another famous trilobite location. The well known Elrathia kingi trilobite is found in spectacular abundance in the Wheeler Shale (Cambrian) of west-central Utah.
Spectacularly preserved trilobite fossils, often showing soft body parts (legs, gills, antennae, etc.) have been found in British Columbia, Canada (the Cambrian Burgess Shale and similar localities); New York State, U.S.A. (Ordovician Walcott-Rust Quarry, near Utica, and the Beecher Trilobite Beds, near Rome); China (Lower Cambrian Maotianshan shales near Chengjiang); Germany (the Devonian Hunsrück Slates near Bundenbach) and, much more rarely, in trilobite-bearing strata in Utah and Ontario.
Trilobites are collected commercially in Russia (especially in the St. Petersburg area), Germany, Morocco's Atlas Mountains, (where a burgeoning trade in faked trilobites is also under way), Utah, Ohio, British Columbia, and in other parts of Canada.
Importance
The study of Paleozoic trilobites in the Welsh-English borders by Niles Eldredge was fundamental in formulating and testing Punctuated Equilibrium as a mechanism of evolution.[32][33][34]
Identification of the 'Atlantic' and 'Pacific' trilobite faunas either side of the Iapetus suture implied the closure of the Iapetus Ocean, thus providing important supporting evidence for Continental Drift and Plate tectonics.
Trilobites have been important in estimating the rate of speciation during the period known as the Cambrian Explosion because they are the most diverse group of metazoans known from the fossil record of the early Cambrian.[35]
Trilobites are excellent stratigraphic markers of the Cambrian period: researchers who find trilobites with alimentary prosopon, and a micropygium, have found Early Cambrian strata.[36] Most of the Cambrian stratigraphy is based on the use of trilobite marker fossils.
Gallery
See also
- Prehistoric life
- List of trilobites
References
- ↑ Cotton, T.J.; Braddy, S.J. (2004), "The phylogeny of arachnomorph arthropods and the origins of the Chelicerata", Transactions of the Royal Society of Edinburgh: Earth Sciences 94: 169–193, doi:
- ↑ 2.0 2.1 Fortey, Richard (2005), "The Lifestyles of the Trilobites", American Scientist. 92: 446-453
- ↑ 3.0 3.1 Fortey, Richard (2000b), "Olenid trilobites: The oldest known chemoautotrophic symbionts?", Proc. Natl. Acad. Sci. USA. 97(12): 6574–6578, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=18664
- ↑ Moore, R.C., ed. (1959), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1, Trilobita, Boulder, CO & Lawrence, KA: The Geological Society of America & The University of Kansas Press, pp. xix + 560 pp., 415 figs., ISBN 0-8137-3015-5
- ↑ 5.0 5.1 Fortey, R.A. (1990), "Ontogeny, Hypostome attachment and Trilobite classification", Palaeontology 33 (3): 529-576, http://palaeontology.palass-pubs.org/pdf/Vol%2033/Pages%20529-576.pdf
- ↑ Ebach, M.C.; McNamara, K.J. (2002), "A systematic revision of the family Harpetidae (Trilobita)", Records of the Western Australian Museum 21: 135–67
- ↑ 7.0 7.1 Rudkin, D.A.; Young, G.A.; Elias, R.J.; Dobrzanske, E.P. (2003), "The World's biggest Trilobite: Isotelus rex new species from the Upper Ordovician of northern Manitoba, Canada", Palaeontology 70 (1): 99–112, http://www.bioone.org/perlserv/?request=get-document&doi=10.1666%2F0022-3360(2003)077%3C0099%3ATWBTIR%3E2.0.CO%3B2&ct=1&SESSID=fabbe2cc5cbdcd233f972db91e24ae28
- ↑ 8.0 8.1 Fortey, R.A.; Hughs, N.C. (1998), "Brood pouches in trilobites", Journal of Paleontology 72: 639–649, http://findarticles.com/p/articles/mi_qa3790/is_199807/ai_n8785182
- ↑ 9.0 9.1 9.2 9.3 Whittington, H.B. (1997a), "Morphology of the Exoskeleton.", in Kaesler, R.L. (ed), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1, Trilobita, revised. Volume 1: Introduction, Order Agnostida, Order Redlichiida., Boulder, CO & Lawrence, KA: The Geological Society of America, Inc. & The University of Kansas, pp. 1-85, ISBN 0-8137-3115-1
- ↑ 10.0 10.1 10.2 10.3 Bruton, D.L.; Haas, W. (2003a), "Making Phacops come alive", in Lane, P.D., Siveter, D.J. & Fortey R.A. (eds.), Special Papers in Palaeontology 70: Trilobites and Their Relatives: Contributions from the Third International Conference, Oxford 2001, Blackwell Publishing & Palaeontological Association, pp. 331-348
- ↑ Nedin, C. (1999), "Anomalocaris predation on nonmineralized and mineralized trilobites", Geology 27 (11): 987–990, doi:, http://geology.geoscienceworld.org/cgi/content/abstract/27/11/987
- ↑ Bruton, D.L.; Nakrem, H.A. (2005), "Enrolment in a Middle Ordovician agnostoid trilobite.", Acta Palaeontologica Polonica 50(3): 441–448, http://www.nhm.uio.no/geomus/homepages/dokumenter/Acta_Pal_Polon_2005_Bruton_&_Nakrem_Agnostids.pdf
- ↑ 13.0 13.1 Clarkson, E.N. (1993), Invertebrate Paleontology and Evolution (4th ed.), New York: Chapman/Hall
- ↑ Knell, R.J.; Fortey, R.A. (2005), "Trilobite spines and beetle horns: sexual selection in the Palaeozoic?", Biology Letters 1: 196-199, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1626209
- ↑ New Scientist magazine (2005), Earliest combatants in sexual contests revealed (published May 28, 2005), http://www.newscientist.com/channel/life/mg18625015.100/
- ↑ Whittington, H.B. (1997c), "The Trilobite Body.", in Kaesler, R.L. (ed), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1, Trilobita, revised. Volume 1: Introduction, Order Agnostida, Order Redlichiida., Boulder, CO & Lawrence, KA: The Geological Society of America, Inc. & The University of Kansas, pp. 137-169, ISBN 0-8137-3115-1
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 Clarkson, E. N. K. (1979), "The Visual System of Trilobites", Palaeontology 22: 1-22
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Clarkson, E.N. (1997), "The Eye, Morphology, Function and Evolution", in Kaesler, R.L. (ed), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1, Trilobita, revised. Volume 1: Introduction, Order Agnostida, Order Redlichiida., Boulder, CO & Lawrence, KA: The Geological Society of America, Inc. & The University of Kansas, pp. 114-132, ISBN 0-8137-3115-1
- ↑ Clarkson, E. N. K.; Levi-Setti, R. L. (1975), "Trilobite eyes and the optics of Descartes and Huygens.", Nature 254: 663-667
- ↑ 20.0 20.1 Bruton, D.L.; Haas, W. (2003b), "The Puzzling Eye of Phacops", in Lane, P.D., Siveter, D.J. & Fortey R.A., Special Papers in Palaeontology 70: Trilobites and Their Relatives: Contributions from the Third International Conference, Oxford 2001, Blackwell Publishing & Palaeontological Association, pp. 349-362
- ↑ Fortey, R.; Chatterton, B. (2003), "A Devonian Trilobite with an Eyeshade", Science 301: 1689
- ↑ Buschbeck, Elke; Ehmer, Birgit; Hoy, Ron (1999), "Chunk Versus Point Sampling: Visual Imaging in a Small Insect", Science 286 (5442): 1178, doi:, PMID 10550059
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 23.9 Chatterton, B.D.E.; Speyer, S.E. (1997), "Ontogeny", in Kaesler, R.L. (ed), Treatise on Invertebrate Paleontology, Part O, Arthropoda 1, Trilobita, revised. Volume 1: Introduction, Order Agnostida, Order Redlichiida., Boulder, CO & Lawrence, KA: The Geological Society of America, Inc. & The University of Kansas, pp. 173-247, ISBN 0-8137-3115-1
- ↑ Lerosey-Aubril, R.; Feist, R. (2005), "First Carboniferous protaspid larvae (Trilobita)", Journal of Paleontology 79: 702-718
- ↑ 25.0 25.1 The Ontogeny of Trilobites by Rudy Lerosey-Aubril Ph.D.
- ↑ 26.0 26.1 Chatterton, B.D.E.; Speyer, S.E. (1989), "Larval ecology, life history strategies, and patterns of extinction and survivorship among Ordovician trilobites", Paleobiology: 118-132
- ↑ 27.0 27.1 Fortey, Richard (2000), Trilobite!, ISBN 0-00-257012-2
- ↑ Lambert, David (1985), The Field Guide to Prehistoric Life, Facts on File Publications, New York: the Diagram Group, ISBN 0-8160-1125-7
- ↑ Baldwin, C. T. (1977), "Rusophycus Morgati: an asaphid produced trace fossil from the Cambro-Ordovician of Brittany and Northwest Spain", Paleontology 51: 411-425.
- ↑ 30.0 30.1 Garlock, T. L.; Isaacson, P. E. (1977), "An occurrance of a cruziana population in the Moyer Ridge Member of the Bloomsberg Formation (Late Silurian)-Snyder County, Pennsylvania.", Paleontology 51: 282-287, http://www.jstor.org/pss/1303607
- ↑ Woolfe, K.J. (1990), "Trace fossils as paleoenvironmental indicators in the Taylor Group (Devonian) of Antarctica", Palaeogeography, Palaeoclimatology, Palaeoecology 80: 301–310, doi:
- ↑ Niles Eldredge and Stephen Jay Gould, 1972. http://www.blackwellpublishing.com/ridley/classictexts/eldredge.asp "Punctuated equilibria: an alternative to phyletic gradualism"] In T.J.M. Schopf, ed., Models in Paleobiology. San Francisco: Freeman Cooper. pp. 82-115. Reprinted in N. Eldredge Time frames. Princeton: Princeton Univ. Press. 1985
- ↑ Ernst Mayr, 1992. "Speciational Evolution or Punctuated Equilibria" In Albert Somit and Steven Peterson The Dynamics of Evolution. New York: Cornell University Press, pp. pp. 25-26.
- ↑ Michael Shermer, 2001. The Borderlands of Science. New York: Oxford University Press.
- ↑ Lieberman, BS (1999), "Testing the Darwinian Legacy of the Cambrian Radiation Using Trilobite Phylogeny and Biogeography", Journal of Paleontology 73 (2)
- ↑ Schnirel, B.L. (2001), Trilobite Evolution and Extinction, Dania, Florida: Graves Museum of Natural History
Further reading
- Levi-Setti, Riccardo (1993), Trilobites, University of Chicago Press.
- Fortey, RA (2001), "Trilobite systematics: The last 75 years", Journal of Paleontology 75: 1141–1151, doi:.
External links
- Gon III, Sam, A Guide to the Orders of Trilobite, http://www.trilobites.info/. (A site with information covering trilobites from all angles. Includes many line drawings and photographs.)
- The Virtual Fossil Museum - Class Trilobita - Including extensive photographs organized by taxonomy and locality.
- The Trilobite papers
- Western Trilobite Association
- Kevin's Trilobite Gallery - a collection of photographs of trilobite fossils
- Canadian trilobite web site: photographs of trilobite fossils
- The Paleontological Society