Tissue engineering
Tissue engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physio-chemical factors to improve or replace biological functions. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e., bone, cartilage, blood vessels, bladder, etc.). Often, the tissues involved require certain mechanical and structural properties for proper functioning. The term has also been applied to efforts to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bioartificial liver). The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells to produce tissues.
A commonly applied definition of tissue engineering, as stated by Langer and Vacanti, is "an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ".[1] Tissue engineering has also been defined as "understanding the principles of tissue growth, and applying this to produce functional replacement tissue for clinical use."[2] A further description goes on to say that an "underlying supposition of tissue engineering is that the employment of natural biology of the system will allow for greater success in developing therapeutic strategies aimed at the replacement, repair, maintenance, and/or enhancement of tissue function."
Powerful developments in the multidisciplinary field of tissue engineering have yielded a novel set of tissue replacement parts and implementation strategies. Scientific advances in biomaterials, stem cells, growth and differentiation factors, and biomimetic environments have created unique opportunities to fabricate tissues in the laboratory from combinations of engineered extracellular matrices ("scaffolds"), cells, and biologically active molecules. Among the major challenges now facing tissue engineering is the need for more complex functionality, as well as both functional and biomechanical stability in laboratory-grown tissues destined for transplantation. The continued success of tissue engineering, and the eventual development of true human replacement parts, will grow from the convergence of engineering and basic research advances in tissue, matrix, growth factor, stem cell, and developmental biology, as well as materials science and bioinformatics.
In 2003, the NSF published a report entitled "The Emergence of Tissue Engineering as a Research Field" [1], which gives a thorough description of the history of this field.
Contents |
Cells

Tissue engineering utilizes living cells as engineering materials. Examples include using living fibroblasts in skin replacement or repair, cartilage repaired with living chondrocytes, or other types of cells used in other ways.
Cells became available as engineering materials when scientists at Geron Corp. discovered how to extend telomeres in 1998, producing immortalized cell lines. Before this, laboratory cultures of healthy, noncancerous mammalian cells would only divide a fixed number of times, up to the Hayflick limit.
Extraction
From fluid tissues such as blood, cells are extracted by bulk methods, usually centrifugation or apheresis. From solid tissues, extraction is more difficult. Usually the tissue is minced, and then digested with the enzymes trypsin or collagenase to remove the extracellular matrix that holds the cells. After that, the cells are free floating, and extracted using centrifugation or apheresis.
Digestion with trypsin is very dependent on temperature. Higher temperatures digest the matrix faster, but create more damage. Collagenase is less temperature dependent, and damages fewer cells, but takes longer and is a more expensive reagent.
Types of cells
Cells are often categorized by their source:
- Autologous cells are obtained from the same individual to which they will be reimplanted. Autologous cells have the fewest problems with rejection and pathogen transmission, however in some cases might not be available. For example in genetic disease suitable autologous cells are not available. Also very ill or elderly persons, as well as patients suffering from severe burns, may not have sufficient quantities of autologous cells to establish useful cell lines. Moreover since this category of cells needs to be harvested from the patient, there are also some concerns related to the necessity of performing such surgical operations that might lead to donor site infection or chronic pain. Autologous cells also must be cultured from samples before they can be used: this takes time, so autologous solutions may not be very quick. Recently there has been a trend towards the use of mesenchymal stem cells from bone marrow and fat. These cells can differentiate into a variety of tissue types, including bone, cartilage, fat, and nerve. A large number of cells can be easily and quickly isolated from fat, thus opening the potential for large numbers of cells to be quickly and easily obtained. Several companies have been founded to capitalize on this technology, the most successful at this time being Cytori Therapeutics.

- Allogenic cells come from the body of a donor of the same species. While there are some ethical constraints to the use of human cells for in vitro studies, the employment of dermal fibroblasts from human foreskin has been demonstrated to be immunologically safe and thus a viable choice for tissue engineering of skin.
- Xenogenic cells are those isolated from individuals of another species. In particular animal cells have been used quite extensively in experiments aimed at the construction of cardiovascular implants.
- Syngenic or isogenic cells are isolated from genetically identical organisms, such as twins, clones, or highly inbred research animal models.
- Primary cells are from an organism.
- Secondary cells are from a cell bank.
- Stem cells (see main article: stem cell) are undifferentiated cells with the ability to divide in culture and give rise to different forms of specialized cells. According to their source stem cells are divided into "adult" and "embryonic" stem cells, the first class being multipotent and the latter mostly pluripotent; some cells are totipotent, in the earliest stages of the embryo. While there is still a large ethical debate related with the use of embryonic stem cells, it is thought that stem cells may be useful for the repair of diseased or damaged tissues, or may be used to grow new organs.
Engineering materials
Cells are often implanted or 'seeded' into an artificial structure capable of supporting three-dimensional tissue formation. These structures, typically called scaffolds, are often critical, both ex vivo as well as in vivo, to recapitulating the in vivo milieu and allowing cells to influence their own microenvironments. Scaffolds usually serve at least one of the following purposes:
- Allow cell attachment and migration
- Deliver and retain cells and biochemical factors
- Enable diffusion of vital cell nutrients and expressed products
- Exert certain mechanical and biological influences to modify the behaviour of the cell phase
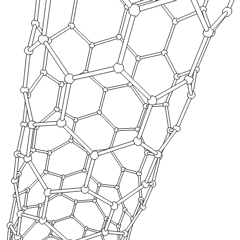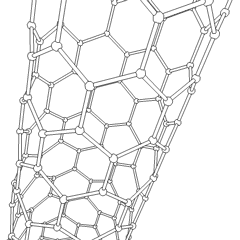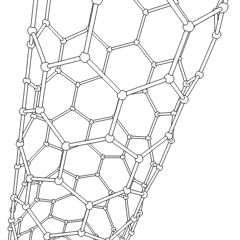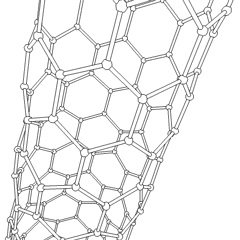
To achieve the goal of tissue reconstruction, scaffolds must meet some specific requirements. A high porosity and an adequate pore size are necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients. Biodegradability is often an essential factor since scaffolds should preferably be absorbed by the surrounding tissues without the necessity of a surgical removal. The rate at which degradation occurs has to coincide as much as possible with the rate of tissue formation: this means that while cells are fabricating their own natural matrix structure around themselves, the scaffold is able to provide structural integrity within the body and eventually it will break down leaving the neotissue, newly formed tissue which will take over the mechanical load. Injectability is also important for clinical uses.
Many different materials (natural and synthetic, biodegradable and permanent) have been investigated. Most of these materials have been known in the medical field before the advent of tissue engineering as a research topic, being already employed as bioresorbable sutures. Examples of these materials are collagen or some linear aliphatic polyesters.
New biomaterials have been engineered to have ideal properties and functional customization: injectability, synthetic manufacture, biocompatibility, non-immunogenicity, transparency, nano-scale fibers, low concentration, resorption rates, etc. PuraMatrix, originating from the MIT labs of Zhang, Rich, Grodzinsky and Langer is one of these new biomimetic scaffold families which has now been commercialized and is impacting clinical tissue engineering.
A commonly used synthetic material is PLA - polylactic acid. This is a polyester which degrades within the human body to form lactic acid, a naturally occurring chemical which is easily removed from the body. Similar materials are polyglycolic acid (PGA) and polycaprolactone (PCL): their degradation mechanism is similar to that of PLA, but they exhibit respectively a faster and a slower rate of degradation compared to PLA.
Scaffolds may also be constructed from natural materials: in particular different derivatives of the extracellular matrix have been studied to evaluate their ability to support cell growth. Proteic materials, such as collagen or fibrin, and polysaccharidic materials, like chitosan or glycosaminoglycans (GAGs), have all proved suitable in terms of cell compatibility, but some issues with potential immunogenicity still remains. Among GAGs hyaluronic acid, possibly in combination with cross linking agents (e.g. glutaraldehyde, water soluble carbodiimide, etc...), is one of the possible choices as scaffold material. Functionalized groups of scaffolds may be useful in the delivery of small molecules (drugs) to specific tissues.
Synthesis of tissue engineering scaffolds
A number of different methods has been described in literature for preparing porous structures to be employed as tissue engineering scaffolds. Each of these techniques presents its own advantages, but none is devoid of drawbacks.
- Nanofiber Self-Assembly: Molecular self-assembly is one of the few methods to create biomaterials with properties similar in scale and chemistry to that of the natural in vivo extracellular matrix (ECM). Moreover, these hydrogel scaffolds have shown superior in vivo toxicology and biocompatibility compared with traditional macroscaffolds and animal-derived materials.
- Textile technologies: these techniques include all the approaches that have been successfully employed for the preparation of non-woven meshes of different polymers. In particular non-woven polyglycolide structures have been tested for tissue engineering applications: such fibrous structures have been found useful to grow different types of cells. The principal drawbacks are related to the difficulties of obtaining high porosity and regular pore size.
- Solvent Casting & Particulate Leaching (SCPL): this approach allows the preparation of porous structures with regular porosity, but with a limited thickness. First the polymer is dissolved into a suitable organic solvent (e.g. polylactic acid could be dissolved into dichloromethane), then the solution is cast into a mold filled with porogen particles. Such porogen can be an inorganic salt like sodium chloride, crystals of saccharose, gelatin spheres or paraffin spheres. The size of the porogen particles will affect the size of the scaffold pores, while the polymer to porogen ratio is directly correlated to the amount of porosity of the final structure. After the polymer solution has been cast the solvent is allowed to fully evaporate, then the composite structure in the mold is immersed in a bath of a liquid suitable for dissolving the porogen: water in case of sodium chloride, saccharose and gelatin or an aliphatic solvent like hexane for paraffin. Once the porogen has been fully dissolved a porous structure is obtained. Other than the small thickness range that can be obtained, another drawback of SCPL lies in its use of organic solvents which must be fully removed to avoid any possible damage to the cells seeded on the scaffold.
- Gas Foaming: to overcome the necessity to use organic solvents and solid porogens a technique using gas as a porogen has been developed. First disc shaped structures made of the desired polymer are prepared by means of compression molding using a heated mold. The discs are then placed in a chamber where are exposed to high pressure CO2 for several days. The pressure inside the chamber is gradually restored to atmospheric levels. During this procedure the pores are formed by the carbon dioxide molecules that abandon the polymer, resulting in a sponge like structure. The main problems related to such a technique are caused by the excessive heat used during compression molding (which prohibits the incorporation of any temperature labile material into the polymer matrix) and by the fact that the pores do not form an interconnected structure.
- Emulsification/Freeze-drying: this technique does not require the use of a solid porogen like SCPL. First a synthetic polymer is dissolved into a suitable solvent (e.g. polylactic acid in dichloromethane) then water is added to the polymeric solution and the two liquids are mixed in order to obtain an emulsion. Before the two phases can separate, the emulsion is cast into a mold and quickly frozen by means of immersion into liquid nitrogen. The frozen emulsion is subsequently freeze-dried to remove the dispersed water and the solvent, thus leaving a solidified, porous polymeric structure. While emulsification and freeze-drying allows a faster preparation if compared to SCPL, since it does not require a time consuming leaching step, it still requires the use of solvents, moreover pore size is relatively small and porosity is often irregular. Freeze-drying by itself is also a commonly employed technique for the fabrication of scaffolds. In particular it is used to prepare collagen sponges: collagen is dissolved into acidic solutions of acetic acid or hydrochloric acid that are cast into a mold, frozen with liquid nitrogen then lyophilized.
- Thermally Induced Phase Separation (TIPS): similar to the previous technique, this phase separation procedure requires the use of a solvent with a low melting point that is easy to sublime. For example dioxane could be used to dissolve polylactic acid, then phase separation is induced through the addition of a small quantity of water: a polymer-rich and a polymer-poor phase are formed. Following cooling below the solvent melting point and some days of vacuum-drying to sublime the solvent a porous scaffold is obtained. Liquid-liquid phase separation presents the same drawbacks of emulsification/freeze-drying.
- CAD/CAM Technologies: since most of the above described approaches are limited when it comes to the control of porosity and pore size, computer assisted design and manufacturing techniques have been introduced to tissue engineering. First a three-dimensional structure is designed using CAD software, then the scaffold is realized by using ink-jet printing of polymer powders or through Fused Deposition Modeling of a polymer melt.[3]
Assembly methods
One of the continuing, persistent problems with tissue engineering is mass transport limitations. Engineered tissues generally lack an initial blood supply, thus making it difficult for any implanted cells to obtain sufficient oxygen and nutrients to survive, and/or function properly.
Self-assembly may play an important role here, both from the perspective of encapsulating cells and proteins, as well as creating scaffolds on the right physical scale for engineered tissue constructs and cellular ingrowth.
It might be possible to print organs, or possibly entire organisms. A recent innovative method of construction uses an ink-jet mechanism to print precise layers of cells in a matrix of thermoreversable gel. Endothelial cells, the cells that line blood vessels, have been printed in a set of stacked rings. When incubated, these fused into a tube.[4] [5]
Tissue culture
In many cases, creation of functional tissues and biological structures in vitro requires extensive culturing to promote survival, growth and inducement of functionality. In general, the basic requirements of cells must be maintained in culture, which include oxygen, pH, humidity, temperature, nutrients and osmotic pressure maintenance.
Tissue engineered cultures also present additional problems in maintaining culture conditions. In standard cell culture, diffusion is often the sole means of nutrient and metabolite transport. However, as a culture becomes larger and more complex, such as the case with engineered organs and whole tissues, other mechanisms must be employed to maintain the culture.
Another issue with tissue culture is introducing the proper factors or stimuli required to induce functionality. In many cases, simple maintenance culture is not sufficient. Growth factors, hormones, specific metabolites or nutrients, chemical and physical stimuli are sometimes required. For example, certain cells respond to changes in oxygen tension as part of their normal development, such as chondrocytes, which must adapt to low oxygen conditions or hypoxia during skeletal development. Others, such as endothelial cells, respond to shear stress from fluid flow, which is encountered in blood vessels.
Bioreactors
In many cases, bioreactors are employed to maintain specific culture conditions. The devices are diverse, with many purpose-built for specific applications. Bioreactors allow for precise and continuous control of culture conditions and also allow for introduction of different stimuli to tissue cultures.
Examples of tissue engineering technologies
- Bioartificial liver device - several research efforts have produced hepatic assist devices utilizing living hepatocytes.
- Artificial pancreas - research involves using islet cells to produce and regulate insulin, particularly in cases of diabetes.
- Artificial bladders - Anthony Atala (Wake Forest University) has successfully implanted artificially grown bladders into seven out of approximately 20 human test subjects as part of a long-term experiment.[6]
- Cartilage - lab-grown tissue was successfully used to repair knee cartilage.[7]
- Doris Taylor's heart in a jar.
- Human Skin Equivalents - artificial skin constructed from fibroblasts embedded in collagen and human keratinocytes seeded on top to form a stratified squamous epithelium.
See also
- Biomedical engineering is a close (and often regarded as parental) field.
- Biological engineering is a broader field that generally encompasses tissue engineering and related fields (e.g. biomaterials).
- Molecular self-assembly
Agencies that support tissue engineering research
- National Institutes of Health
- National Science Foundation
- National Research Council of Canada
- Department of Biomolecular Engineering, Melbourne University
References
- ↑ Langer, R & Vacanti JP, Tissue engineering. Science 260, 920-6; 1993.
- ↑ MacArthur, B. D. & Oreffo, R.O.C. (2005). "Bridging the gap". Nature 433, 19.
- ↑ Ma, Peter X., and Jennifer Elisseeff, eds. Scaffolding in Tissue Engineering. New York: C R C P LLC, 2005.
- ↑ Mironov, V., Boland, T., Trusk, T., Forgacs, G. & Markwald, R.R., Organ printing: computer-aided jet-based 3D tissue engineering. Trends in Biotechnology 21, 157-61; 2003.
- ↑ Ma, Peter X., and Jennifer Elisseeff, eds. Scaffolding in Tissue Engineering. New York: C R C P LLC, 2005.
- ↑ Doctors grow organs from patients' own cells, CNN, April 3, 2006
- ↑ Lab-grown cartilage fixes damaged knees - health - 05 July 2006 - New Scientist Space
-
- Davis, M.E., et al., Injectable Self-Assembling Peptide Nanofibers Create Intramyocardial Microenvironments for Endothelial Cells. Circulation 111:442-450 (2005).
- Ma, Peter X.: Scaffolds for tissue fabrication - Materials Today, May 2004, 30-40.
- Holmes, T.C., et al., Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. PNAS USA 97 :6728 (2000).
- Semino, C.E., et al., Entrapment of migrating hippocampal neural cells in 3D peptide nanofiber scaffold. Tissue Engineering 10:643 (2004).
- Mikos, A. G. & Temenoff J. S. (2000). "Formation of highly porous biodegradable scaffolds for tissue engineering". Electronic Journal of Biotechnology 3, 114-119. URL accessed on April 28, 2006.
-
- National Science Foundation (U.S.A.) (2004). "The Emergence of Tissue Engineering as a Research Field". URL accessed on April 28, 2006.
- Nerem, R.M., in Principles of Tissue Engineering. Lanza, Langer and J Vacanti (eds), 2000.
External links
Organizations
- Clinical Tissue Engineering Center State of Ohio Initiative for Tissue Engineering (National Center for Regenerative Medicine)
- Pittsburgh Tissue Engineering Initiative
- Tissue Engineering Society of Malaysia Pages
- Tissue Engineering and Regenerative Medicine International Society
Directories and Repositories
- Malaysia Tissue Engineering Laboratory Pages
- Tissue engineering articles and information
- Tissue Engineering Pages
Research Initiatives
- Institute for Chemical Process and Environmental Technology Tissue engineered (TE) corneas
- Organ Printing Multi-site NSF-funded initiative
- Team Research Tissue Engineering and Medical Research Programmes
|
||||||||||||||||||||||||||