Tin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

The alchemical symbol for tin. Also used as the glyph for Jupiter.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tin is a chemical element with the symbol Sn (Latin: Stannum) and atomic number 50. Tin is obtained chiefly from the mineral cassiterite, where it occurs as an oxide, SnO2. This silvery, malleable poor metal that is not easily oxidized in air, and is used to coat other metals to prevent corrosion. It is found in many alloys, most notably bronze. Pewter alloys contain from 85% up to 99% tin.
Contents |
Characteristics
Physical
Tin is a malleable, ductile, and highly crystalline silvery-white metal. Tin is malleable at ordinary temperatures but is brittle when it is cooled. When a bar of tin is bent, a crackling sound known as the tin cry can be heard due to the twinning of the crystals.
Chemical
Tin resists corrosion from distilled, sea and soft tap water, but can be attacked by strong acids, alkalis, and by acid salts. Tin can be highly polished and is used as a protective coat for other metals in order to prevent corrosion or other chemical action. Tin acts as a catalyst when oxygen is in solution and helps accelerate chemical attack. Tin forms the dioxide SnO2 (cassiterite) when it is heated in the presence of air. SnO2, in turn, is feebly acidic and forms stannate (SnO32-) salts with basic oxides. There are also stanates with the structure [Sn(OH)6]2-, like K2[Sn(OH)6], although the free stanic acid H2[Sn(OH)6] is unknown. This metal combines directly with chlorine forming tin(IV) chloride, while reacting tin with hydrochloric acid in water gives tin(II) chloride and hydrogen. Several other compounds of tin exist in the oxydation state +2 and +4, for example the tin(II) sulfide and the tin(IV) sulfide Mosaic gold. For the hydrogen compounds this is not true, here only the oxidation state 4 is stable, the stannane (SnH4).[1]
Allotropes
Tin's chemical properties fall between those of metals and non-metals, just as the semiconductors silicon and germanium do. Tin has two allotropes at normal pressure and temperature: gray tin and white tin. A third allotrope, called brittle tin, exists at temperatures above 161 °C.
Below 13.2 °C, it exists as gray or alpha tin, which has a cubic crystal structure similar to silicon and germanium. Gray tin has no metallic properties at all, is a dull-gray powdery material, and has few uses, other than a few specialized semiconductor applications.
Although the transformation temperature is 13.2 °C, the change does not take place unless the metal is of high purity, and only when the exposure temperature is well below 0 °C.[2] This process is known as tin disease or tin pest. Tin pest was a particular problem in northern Europe in the 18th century as organ pipes made of tin alloy would sometimes be affected during long cold winters. Some sources also say that during Napoleon's Russian campaign of 1812, the temperatures became so cold that the tin buttons on the soldiers' uniforms disintegrated, contributing to the defeat of the Grande Armée. The veracity of this story is debatable, because the transformation to gray tin often takes a reasonably long time.[3] Commercial grades of tin (99.8%) resist transformation because of the inhibiting effect of the small amounts of bismuth, antimony, lead, and silver present as impurities. Alloying elements such as copper, antimony, bismuth, cadmium, and silver increase its hardness. Tin tends rather easily to form hard, brittle intermetallic phases, which are often undesirable. It does not form wide solid solution ranges in other metals in general, and there are few elements that have appreciable solid solubility in tin. Simple eutectic systems,however, occur with bismuth, gallium, lead, thallium, and zinc.[2]
Isotopes
Tin is the element with the greatest number of stable isotopes (ten), which is probably related to the fact that 50 is a "magic number" of protons. 28 additional unstable isotopes are known, including the "doubly magic" tin-100 (100Sn) (discovered in 1994).[4] tin can also be gray
Applications
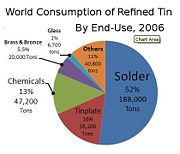
In 2006, the categories of tin use were solder (52%), tinplate (16%), chemicals (13%), brass and bronze (5.5%), glass (2%), and variety of other applications (11%) [5]
Metal and Alloy
Tin is used by itself, or in combination with other elements for a wide variety of useful alloys.

- Tin is used extensively for alloys, some important tin alloys are bronze, bell metal, Babbitt metal, die casting alloy, pewter, phosphor bronze, soft solder, and White metal.
- Today tin is primarily used for solders for joining pipes or electric circuits, and increasingly so with the high demand for electonics on the one hand, and the replacement of alloys with lead, due to its toxicity. Though replacing lead has many problems, including a higher melting point, replacement alloys are rapidly being found. [6]
- Tin is also replacing lead in bearing alloys, in glass-making, and in a wide range of tin chemical applications.
- Tin bonds readily to iron, and is used for coating lead or zinc and steel to prevent corrosion.
- Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin. Speakers of British English call them "tins"; Americans call them "cans" or "tin cans". One thus-derived use of the slang term "tinnie" or "tinny" means "can of beer". The tin whistle is so called because it was first mass-produced in tin-plated steel.
- Tin foil was once a common wrapping material for foods and drugs; replaced in the early 20th century by the use of aluminium foil, which is now commonly referred to as tin foil. Hence one use of the slang term "tinnie" or "tinny" for a small pipe for use of a drug such as cannabis or for a can of beer.
- Most metal pipes in a pipe organ are made of varying amounts of a tin/lead alloy, with 50%/50% being the most common. The amount of tin in the pipe defines the pipe's tone, since tin is the most tonally resonant of all metals. When a tin/lead alloy cools, the lead cools slightly faster and makes a mottled or spotted effect. This metal alloy is referred to as spotted metal.
- Window glass is most often made via floating molten glass on top of molten tin (creating float glass) in order to make a flat surface (this is called the "Pilkington process").

- Tin becomes a superconductor below 3.72 K. In fact, tin was one of the first superconductors to be studied; the Meissner effect, one of the characteristic features of superconductors, was first discovered in superconducting tin crystals. The niobium-tin compound Nb3Sn is commercially used as wires for superconducting magnets, due to the material's high critical temperature (18 K) and critical magnetic field (25 T). A superconducting magnet weighing only a couple of kilograms is capable of producing magnetic fields comparable to a conventional electromagnet weighing tons.
Compounds
- For a long time the antifouling property of organotin compounds was used for the preservation of wood and to prevent growth of marine organisms on ships. Tributyltin was used as additive for ship paint. The use declined after organotin compounds where recognised as persistent organic pollutants with a extremely high toxicity for some marine organism, for example dog whelk.[7] The EU banned the use of organotin compounds in 2003.[8]
- The most important salt formed is stannous chloride, which has found use as a reducing agent and as a mordant in the calico printing process. Electrically conductive coatings are produced when tin salts are sprayed onto glass. These coatings have been used in panel lighting and in the production of frost-free windshields.
- Tin is added to some dental care products[9] as stannous fluoride (SnF2). Stannous fluoride can be mixed with calcium abrasives while the more common sodium fluoride gradually becomes biologically inactive combined with calcium.[10] It has also been shown to be more effective than sodium fluoride in controlling gingivitis.[11]
Compounds
For discussion of Stannate compounds (SnO32−) see Stannate. For Stannite (SnO2−) see Stannite. See also Stannous hydroxide (Sn(OH)2), Stannic acid (Stannic Hydroxide - Sn(OH)4), Tin dioxide (Stannic Oxide - SnO2), Tin(II) oxide (Stannous Oxide - SnO), Tin(II) chloride (SnCl2), Tin(IV) chloride (SnCl4)
See also Category:Tin compounds
History
Tin (Old English: tin, Old Latin: plumbum candidum ("white lead"), Old German: tsin, Late Latin: stannum) is one of the earliest metals known and was used as a component of bronze from antiquity.[12]Because of its hardening effect on copper, tin was used in bronze implements as early as 3,500 BC. A shipwreck at Uluburun, Turkey dating to 1336 BC contains a shipment of tin, perhaps originating in Afghanistan.[13] European tin mining is believed to have started in Cornwall and Devon (esp. Dartmoor) in Classical times, and a thriving tin trade developed with the civilizations of the Mediterranean.[14][15] However the lone metal was not used until about 600 BC. The last Cornish tin mine, at South Crofty near Camborne, closed in 1998 bringing 4,000 years of mining in Cornwall to an end, but as of 2007 increased demand from China may lead to its re-opening.[16]

The word "tin" has cognates in many Germanic and Celtic languages. The American Heritage Dictionary speculates that the word was borrowed from a pre-Indo-European language. The later name "stannum" and its Romance derivatives come from the lead-silver alloy of the same name for the finding of the latter in ores; the former "stagnum" was the word for a stale pool or puddle.
In modern times, the word "tin" is often improperly used as a generic phrase for any silvery metal that comes in sheets. Most everyday materials that are commonly called "tin", such as aluminium foil, beverage cans, corrugated building sheathing and tin cans, are actually made of steel or aluminium, although tin cans (tinned cans) do contain a thin coating of tin to inhibit rust. Likewise, so-called "tin toys" are usually made of steel, and may or may not have a coating of tin to inhibit rust. The original Ford Model T was known colloquially as the Tin Lizzy.
Historical Cornwall was the major tin producer, this changed after large amounts of tin have been found in the Bolivian tin belt and the east Asian tin belt stretching from China through Thailand and Laos to Malaya and Indonesia. The tin produceres founded in 1931 the International Tin Comittee followed in 1956 by the International Tin Council a institution to control the tin market. After the collapse of the market in October 1985 the price for tin nearly halved.
Occurrence
See also Category:Tin minerals


Tin is a relatively scarce element with an abundance in the Earth's crust of about 2 ppm, compared with 94 ppm for zinc, 63 ppm for copper, and 12 ppm for lead. Tin does not occur naturally by itself, and must be extracted from a base compound, usually cassiterite(SnO2), the only commercially important source of tin, although small quantities of tin are recovered from complex sulfides such as stannite, cylindrite, franckeite, canfieldite, and teallite. Minerals with tin are almost always in association with granite rock, which when contain the mineral, have a 1% tin oxide content[17]Due to the higher specific gravity of tin and its resistance to corrosion, about 80% of mined tin is from secondary deposits found downstream from the primary lodes. Tin is often recovered from granules washed downstream in the past and deposited in valleys or under sea. The most economical ways of mining tin are through dredging, hydraulic methods or open cast mining. Most of the world's tin is produced from placer deposits, which may contain as little as 0.015% tin. Secondary, or scrap, tin is also an important source of the metal.
It was estimated in Jan 2008 that there were 6.1 million tons of economically recoverable primary reserves, from a known base reserve of 11 million tons. Below are the nations with the 10 largest known reserves.
| Country | Reserves | Reserve Base |
|---|---|---|
| China | 1,700,000 | 3,500,000 |
| Malaysia | 1,000,000 | 1,200,000 |
| Peru | 710,000 | 1,000,000 |
| Indonesia | 800,000 | 900,000 |
| Brazil | 540,000 | 2,500,000 |
| Bolivia | 450,000 | 900,000 |
| Russia | 300,000 | 350,000 |
| Other | 180,000 | 200,000 |
| Thailand | 170,000 | 250,000 |
| Australia | 150,000 | 300,000 |
| Congo-Kinshasa | NA | NA |
It is estimated that, at current consumption rates and technologies, the Earth will run out of tin that can be mined in 40 years.[18] However Lester Brown has suggested tin could run out within 20 years based on an extremely conservative extrapolation of 2% growth per year.[19] Estimates of tin production have historically varied with the dynamics of economic feasibility and the development of mining technologies.
| Estimated Economically Recoverable World Tin Reserves (tons)[20] |
|
|---|---|
| 1965 | 4265 |
| 1970 | 3930 |
| 1975 | 9060 |
| 1980 | 9100 |
| 1985 | 3060 |
| 1990 | 7100 |
| 2008 | 6100[21] |
The recovery of tin through secondary production, or recycling of scrap tin, is increasing rapidly. While the United States has neither mined since 1993 nor smelted tin since 1989, it was the largest secondary producer, recycling nearly 14,000 tons in 2006[22].
| Cumulative Global Tin Production (tons)[23] | ||
|---|---|---|
| 1850 | 2000 | 2000 |
| 1925 | 5500 | 7500 |
| 1970 | 7659 | 15159 |
| 2006 | 8274 | 23433 |
Tasmania hosts some deposits of historical importance, most notably Mount Bischoff and Renison Bell. New deposits are also reported to be in southern Mongolia.
Production
Tin is produced by reducing the ore with coal in a reverberatory furnace. This metal is a relatively scarce element with an abundance in the Earth's crust of about 2 ppm, compared with 94 ppm for zinc, 63 ppm for copper, and 12 ppm for lead. Most of the world's tin is produced from placer deposits. The only mineral of commercial importance as a source of tin is cassiterite (SnO2), although small quantities of tin are recovered from complex sulfides such as stannite, cylindrite, franckeite, canfieldite, and teallite. Secondary, or scrap, tin is also an important source of the metal.
Mining and Smelting
| Country | Mine Production | Smelter Production |
|---|---|---|
| China | 114,300 | 129,400 |
| Indonesia | 117,500 | 80,933 |
| Peru | 38,470 | 40,495 |
| Bolivia | 17,669 | 13,500 |
| Thailand | 225 | 27,540 |
| Malaysia | 2,398 | 23,000 |
| Belgium | 0 | 8,000 |
| Russia | 5,000 | 5,500 |
| Congo-Kinshasa ('08) | 15,000 | 0 |
In 2006, total worldwide tin mine production was 321,000 tons, and smelter production was 340,000 tons. From its production level of 186,300 tons in 1991, around where it had hovered for the previous decades, production of tin shot up 89%, to 351,800 tons in 2005. Most of the increase came from China and Indonesia, with the largest spike in 2004-5, when it increased 23%. While in the 1970s Malaysia was the largest producer, with around a third of world production, it has steadily fallen, and now remains a major smelter and market center.
In 2007, the People's Republic of China was the largest producer of tin, where the tin deposits are concentrated in the southeast Yunnan tin belt,[25] with 43% of the world's share, followed by Indonesia, with an almost equal share, and Peru at a distant third, reports the USGS.[21]
After the discovery of tin in what is now Bisie, North Kivu in the Democratic Republic of Congo in 2002, illegal production has increased there to around 15,000 tons[26]. This is largely fueling the ongoing and recent conflicts there, as well as affecting international markets.
Shown is a table of the countries with the largest mine production and the largest smelter output (estimates vary between USGS[27] and The British Geological Survey, the latter of which was chosen because it indicates that the most recent statistics are not estimates, and estimates match more closely with other estimates found for Congo-Kinshasa).
Industry
The ten largest companies produced most of world's tin in 2007. It is not clear which of these companies include tin smelted from the mine at Bisie, Congo-Kinshasa, which is controlled by a renegade militia and produces 15,000 tons. Most of the world's tin is traded on the London Metal Exchange (LME), from 8 countries, under 17 brands[28]. Prices of tin were at $11,900 per ton as of Nov 24, 2008. Prices reached an all time high of nearly $25,000 per ton in May 2008, largely because of the effect of the decrease of tin production from Indonesia, and have been volatile since because of reliance from mining in Congo-Kinshasa[29].
| Company | 2006 | 2007 | %Change |
|---|---|---|---|
| Yunnan Tin (China) | 52,339 | 61,129 | 16.7 |
| PT Timah (Indonesia) | 44,689 | 58,325 | 30.5 |
| Minsur (Peru) | 40,977 | 35,940 | -12.3 |
| Malay (China) | 52,339 | 61,129 | 16.7 |
| Malaysia Smelting Corp (Malaysia) | 22,850 | 25,471 | 11.5 |
| Thaisarco (Thailand) | 27,828 | 19,826 | -28.8 |
| Yunnan Chengfeng (China) | 21,765 | 18,000 | -17.3 |
| Liuzhou China Tin (China) | 13,499 | 13,193 | -2.3 |
| EM Vinto (Bolivia) | 11,804 | 9,448 | -20.0 |
| Metallo Chimique (Belgium) | 8,049 | 8,342 | 4.0 |
| Gold Bell Group (China) | 4,696 | 8,000 | 70.4 |
Precautions
Tin plays no known natural biological role in humans, and possible health effects of tin are a subject of dispute. Tin itself is not toxic but most tin salts are.
Triorganotins are very toxic. Tri-n-alkyltins are phytotoxic and depending on the organic groups, they can be powerful bactericides and fungicides. Other triorganotins are used as miticides and acaricides.
See also
- International Tin Council
- Stannary
- Tinning
- Cassiterides (the mythical Tin Islands)
- Tin pest
- Whisker (metallurgy) (tin whiskers)
- Terne
References
- ↑ Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils; (1985). "Tin" (in German). Lehrbuch der Anorganischen Chemie (91–100 ed.). Walter de Gruyter. pp. 793–800. ISBN 3110075113.
- ↑ 2.0 2.1 Schwartz, Mel (2002). "Tin and Alloys, Properties". Encyclopedia of Materials, Parts and Finishes (2nd ed.). CRC Press. ISBN 1566766613.
- ↑ Le Coureur, Penny; Burreson, Jay (2004). Napoleon's Buttons: 17 Molecules that Changed History. New York: Penguin Group USA.
- ↑ Walker, Phil (1994). "Doubly Magic Discovery of Tin-100". Physics World 7 (June). http://physicsworldarchive.iop.org/index.cfm?action=summary&doc=7%2F6%2Fphwv7i6a24%40pwa-xml&qt=.
- ↑ "ITRI. Tin Use Survey 2007". ITRI. Retrieved on 2008-11-21.
- ↑ Black, Harvey. Getting the Lead out of Electronics. Environmental Health Perspectives. v.113(10); Oct 2005
- ↑ Eisler, Ronald. "Tin Hazards To Fish, Wildlife, and Invertebrates: A Synoptic Review". U.S. Fish and Wildlife Service Patuxent Wildlife Research Center.
- ↑ REGULATION (EC) No 782/2003 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 14 April 2003 on the prohibition of organotin compounds on ships
- ↑ Crest Pro Health, Colgate Gel-Kam
- ↑ Hattab, F. (April 1989). "The State of Fluorides in Toothpastes.". Journal of Dentistry 17 (2): 47–54. doi:. PMID 2732364.
- ↑ "The clinical effect of a stabilized stannous fluoride dentifrice on plaque formation, gingivitis and gingival bleeding: a six-month study.". The Journal of Clinical Dentistry 6 (Special Issue): 54–58. 1995. PMID 8593194.
- ↑ Johann Beckmann, William Francis, William Johnston, John William Griffith (1846). A History of Inventions, Discoveries, and Origins. H.G. Bohn. pp. 57–68. http://books.google.de/books?id=qGMSAAAAIAAJ.
- ↑ Martin Ewans. Afghanistan. Harper Collins, 2001. ISBN 0-06-050508-7
- ↑ Wake, H. (2006-04-07). "Why Claudius invaded Britain" (HTML). Etrusia - Roman History. Retrieved on 2007-01-12.
- ↑ McKeown, James (1999-01). "The Romano-British Amphora Trade to 43 A.D: An Overview" (HTML). Retrieved on 2007-01-12.
- ↑ Hickman, Leo (2007-11-30). "The Return of Tin" (HTML). Retrieved on 2007-12-04.
- ↑ International Tin Research Institute. Tin: From Ore to Ingot.1991. http://www.itri.co.uk/pooled/articles/BF_TECHART/view.asp?Q=BF_TECHART_230527
- ↑ "How Long Will it Last?". New Scientist 194 (2605): 38–39. May 26, 2007. ISSN 4079 0262 4079.
- ↑ Brown, Lester Plan B 2.0, New York: W.W. Norton, 2006. p. 109
- ↑ International Tin Research Institute. Tin: From Ore to Ingot.1991. http://www.itri.co.uk/pooled/articles/BF_TECHART/view.asp?Q=BF_TECHART_230527
- ↑ 21.0 21.1 Carlin, Jr., James F.. "Mineral Commodity Summary 2008: Tin". United States Geological Survey.
- ↑ You must specify title = and url = when using {{cite web}}.Carlin, Jr., James F.. "". United States Geological Survey. Retrieved on 2008-11-23.
- ↑ ITRI. Long-term Trends in Tin-in-Concentrate Production, 1970-2006.
- ↑ World Mineral Production; 2002-06. British Geological Survey. Pg. 89. http://www.bgs.ac.uk/mineralsuk/downloads/wmp_2002_2006.pdf
- ↑ "Classification and type association of tin deposits in Southeast Yunnan Tin Belt". Chinese Journal of Geochemistry 10 (1): 21–35. 1991. doi:.
- ↑ The Spoils: Congo's Riches, Looted by Renegade Troops. New York Times. Nov 15, 2008. http://www.nytimes.com/2008/11/16/world/africa/16congo.html?ref=africa
- ↑ 2006 Minreals Yearbook. Tin. http://minerals.usgs.gov/minerals/pubs/commodity/tin/myb1-2006-tin
- ↑ International Tin Research Institute. LME Tin Brands. http://www.itri.co.uk/pooled/articles/BF_TECHART/view.asp?Q=BF_TECHART_303032
- ↑ ANALYSIS-Tin price spike shows Congo's growing origin role. Reuters. http://www.alertnet.org/thenews/newsdesk/LU661455.htm
- ↑ International Tin Research Institute. Top Ten Tin Producing Companies. http://www.itri.co.uk/pooled/articles/BF_TECHART/view.asp?Q=BF_TECHART_285697
External links
- WebElements.com – Tin
- Theodore Gray's Wooden Periodic Table Table: Tin samples and castings
- Base Metals: Tin
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||