Tannin

Tannins are astringent, bitter plant polyphenols that either bind and precipitate or shrink proteins. The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of red wine, strong tea, or an unripened fruit.[1] The term tannin refers to the use of tannins in tanning animal hides into leather; however, the term is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with proteins and other macromolecules. Tannins have molecular weights ranging from 500 to over 3,000.[2] Tannins are incompatible with alkalis, gelatin, heavy metals, iron, lime water, metallic salts, strong oxidizing agents and zinc sulfate.
| Base Unit: | 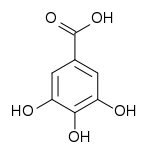 |
 |
|---|---|---|
| Class/Polymer: | Hydrolyzable Tannins | Condensed Tannins |
Tannins are usually divided into hydrolyzable tannins and condensed tannins (proanthocyanidins).
Contents |
Occurrence
Tannins are distributed all over the plant kingdom. They are commonly found in both gymnosperms as well as angiosperms. In terms of location of the tannins in a plant, they are mainly located in the vacuoles or surface wax of the plants. These sites are where tannins do not interfere with plant metabolism, and it is only after cell breakdown and death that the tannins are active in metabolic effects. Tannins are found in leaf tissues, bud tissues, seed tissues, root tissues and stem tissues. An example of the location of the tannins in the stem tissue is that they are often found in the growth areas of trees, such as the secondary phloem and xylem and the layer between the cortex and epidermis. Tannins may help regulate the growth of these tissues. They are also found in the heartwood of conifers and may play a role in inhibiting microbial activity, thus resulting in the natural durability of the wood.[3] However, there may be a loss in the bioavailability of tannins in plants due to birds, pests, and other pathogens.[4]
The leaching of tannins from the decaying leaves of vegetation adjoining a stream may produce what is known as a blackwater river.
Hydrolyzable tannins
At the center of a hydrolyzable tannin molecule, there is a carbohydrate (usually D-glucose). The hydroxyl groups of the carbohydrate are partially or totally esterified with phenolic groups such as gallic acid (in gallotannins) or ellagic acid (in ellagitannins). Hydrolyzable tannins are hydrolyzed by weak acids or weak bases to produce carbohydrate and phenolic acids.
Examples of gallotannins are the gallic acid esters of glucose in tannic acid (C76H52O46), found in the leaves and bark of many plant species.
Condensed tannins
Condensed tannins, also known as proanthocyanidins, are polymers of 2 to 50 (or more) flavonoid units that are joined by carbon-carbon bonds, which are not susceptible to being cleaved by hydrolysis. While hydrolyzable tannins and most condensed tannins are water soluble, some very large condensed tannins are insoluble.
Foods with tannins
Tea

The tea plant (Camellia sinensis) is an example of a plant said to have a naturally high tannin content. When any type of tea leaf is steeped in hot water it brews a "tart" (astringent) flavor that is characteristic of tannins. This is due to the catechins and other flavonoids. Tea "tannins" are chemically distinct from other types of plant tannins such as tannic acid[5] and tea extracts have been reported to contain no tannic acid.[6] Black tea and peppermint tea are more inhibitory of iron than herb teas like chamomile, vervain, lime flower and pennyroyal.[7]
Wine
- See also: Phenolic compounds in wine

Tannins (mainly condensed tannins) are also found in wine, particularly red wine. Tannins in wine can come from many sources and the tactile properties differ depending on the source. Tannins in grape skins and seeds (the latter being especially harsh) tend to be more noticeable in red wines, which are fermented while in contact with the skins and seeds to extract the colour from the skins. The stems of the grape bunches also contain tannins, and will contribute tannins if the bunches are not de-stemmed before pressing and fermentation. Tannins extracted from grapes are condensed tannins, which are polymers of proanthocyanidin monomers. Hydrolysable tannins are extracted from the oak wood the wine is aged in. Hydrolysable tannins are more easily oxidised than condensed tannins.
Modern winemakers take great care to minimize undesirable tannins from seeds by crushing grapes gently when extracting their juice, to avoid crushing the seeds. Pressing the grapes further results in press wine which is more tannic and might be kept separately. De-stemming is also widely practiced. Wines can also take on tannins if matured in oak or wood casks with a high tannin content. Tannins play an important role in preventing oxidation in aging wine and appear to polymerize and make up a major portion of the sediment in wine.
Recently, a study in wine production and consumption has shown that tannins, in the form of proanthocyanidins, have a beneficial effect on vascular health. The study showed that tannins suppressed production of the peptide responsible for hardening arteries. To support their findings, the study also points out that wines from the regions of southwest France and Sardinia are particularly rich in proanthocyanidins, and that these regions also produce populations with longer life spans.[8]
Effects of tannins on the drinkability and aging potential of wine
Tannins in wine have been described, particularly by novice drinkers, as having the effect of making wine difficult to drink compared to a wine with a lower level of tannins. Tannins can be described as leaving a dry and puckered feeling with a "furriness" in the mouth that can be compared to a stewed tea, which is also very tannic. This effect is particularly profound when drinking tannic wines without the benefit of food.
Many oenophiles see natural tannins (found particularly in varietals such as Cabernet Sauvignon and often accentuated by heavy oak barrel aging) as a sign of potential longevity and ageability. As tannic wines age, the tannins begin to decompose and the wine mellows and improves with age, with the tannic "backbone" helping the wine survive for as long as 40 years or more. A strongly tannic wine is also well-matched to very fatty food courses, in particular steaks; the tannins help break down the fat, with a salutary impact on both the wine and the steak. In many regions (such as in Bordeaux), tannic grapes such as Cabernet Sauvignon are blended with lower-tannin grapes such as Merlot or Cabernet Franc, diluting the tannic characteristics. Wines that are vinified to be drunk young typically have lower tannin levels.
Fruits
Pomegranates


Pomegranates contain a diverse array of tannins, particularly hydrolysable tannins. The most abundant of pomegranate tannins are called punicalagins. Punicalagins have a molecular weight of 1038 and are the largest molecule found intact in rat plasma after oral ingestion[9] and were found to show no toxic effects in rats who were given a 6% diet of punicalagins for 37 days.[10] Punicalagins are also found to be the major component responsible for pomegranate juice's antioxidant and health benefits.[11]
Several dietary supplements and nutritional ingredients are available that contain extracts of whole pomegranate and/or are standardized to punicalagins, the marker compound of pomegranate. Extracts of pomegranate are also Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration. It has been recommended to look for pomegranate ingredients that mimic the polyphenol ratio of the fruit, as potent synergistic effects have been observed in 'natural spectrum' extracts, especially pomegranate concentrate normalized to punicalagins.[12]
Persimmons
Some persimmons are highly astringent and therefore inedible when they are not extremely ripe (specifically the Korean, American, and Hachiya or Japanese). This is due to the high level of tannins, and if eaten by humans (and many other animals), the mouth will become completely dry, yet the saliva glands will continue to secrete saliva which cannot affect the tannin-laced food. Areca Catechu also contains tannin which contributes to its antibacterial properties.
Berries

Most berries, such as cranberries,[13] strawberries and blueberries,[14] contain both hydrolyzable and condensed tannins.
Smoked foods
Tannins from the wood of mesquite, cherry, oak and other woods used in smoking are present on the surface of smoked fish and meat.
Beer
In addition to the alpha acids extracted from hops to provide bitterness in beer, tannins are also present as well. In most cases, the presence of tannins is considered a flaw. However, in some styles the presence of this astringency is acceptable or even desired, as, for example, in a Flanders red ale.
Citrus, fruit juices
Although citrus fruits do not themselves contain tannins, orange-colored juices often contain food dyes with tannins. Apple juice, grape juices and berry juices are all high in tannins. Sometimes tannins are even added to juices and ciders to create a more astringent feel to the taste.
Condiments
Cloves, tarragon, cumin, thyme, vanilla, and cinnamon all contain tannins.[15]
Legumes
Most legumes contain tannins. Red-colored beans contain the most tannins, and white-colored beans have the least. Chickpeas (also known as garbanzo beans) have a smaller amount of tannins.[16]
Chocolate
Tannin is also found in chocolate. The usual concentration is around 10mg per ml in the liquid form. You would have to eat 100 bars of chocolate to consume the equivalent amount found in a bottle of wine.[17]
Nutrition
Tannins have traditionally been considered antinutritional but it is now known that their beneficial or antinutritional properties depend upon their chemical structure and dosage. The new technologies used to analyze molecular and chemical structures have shown that a division into condensed and hydrolysable tannins is far too simplistic.[18] Recent studies have demonstrated that products containing chestnut tannins included at low dosages (0.15-0.2 %) in the diet can improve wellbeing.[19] Studies on chestnut tannins have shown beneficial effects on silage quality in the round bale silages, in particular reducing ammonia and NPN (non protein nitrogen) in the lowest wilting level.[20] Improved fermentability of soya meal nitrogen in the rumen has also been reported by Mathieu F and Jouany JP (1993).[21] Studies by Gonzalez S. et al (2002)[22] on in vitro ammonia release and dry matter degradation of soybean meal comparing three different types of tannins (quebracho, acacia and chestnut) demonstrated that chestnut tannins are more efficient in protecting soybean meal from in vitro degradation by rumen bacteria.
If ingested in excessive quantities, tannins inhibit the absorption of minerals such as iron and calcium which may, if prolonged, lead to anemia or osteoporosis.[23] This is because tannins are metal ion chelators, and tannin-chelated metal ions are not bioavailable. This may not be bad for someone with an infection, as iron is mopped up by the immune system to keep microorganisms from properly multiplying. Tannins have been shown to precipitate proteins,[2] which inhibits in some ruminant animals the absorption of nutrients from high-tannin grains such as sorghum. Tannins only reduce the bioavailability of plant sources of iron, also known as non-heme. Animal sources, or heme iron absorption will not be affected by tannins. Tannic acid does not affect absorption of other trace minerals such as zinc, copper, and manganese in rats.[24]
Tannins are phenolic compounds and interfere with iron absorption through a complex formation with iron when it is in the gastrointestinal lumen which decreases the bioavailability of iron. There is an important difference in the way in which the phenolic compounds interact with different hydroxylation patterns (gallic acid, catechin, chlorogenic acid) and the effect on iron absorption. The content of the iron-binding galloyl groups may be the major determinant of the inhibitory effect of phenolic compounds. However, condensed tannins do not interfere with iron absorption.[23]
In order to prevent these problems, it is advised to drink tea and coffee between meals, not during. Foods rich in vitamin C help neutralize tannin's effects on iron absorption. Adding lemon juice to tea will reduce the negative effect of tannins in iron absorption as well. Adding milk to coffee and tea has very little to no influence on the inhibitory effect of tannins.[25]
In sensitive individuals, a large intake of tannins may cause bowel irritation, kidney irritation, liver damage, irritation of the stomach and gastrointestinal pain. With the exception of tea, long-term and/or excessive use of herbs containing high concentrations of tannins is not recommended. A correlation has been made between esophogeal or nasal cancer in humans and regular consumption of certain herbs with high tannin concentrations.[26]
Uses
Tannins are an important ingredient in the process of tanning leather. Oak bark has traditionally been the primary source of tannery tannin, though inorganic tanning agents are also in use today.
Tannins may be employed medicinally in antidiarrheal, hemostatic, and antihemorrhoidal compounds
The anti-inflammatory effect of tannins help control all indiccations of gastritis, esophagitis, enteritis, and irritating bowel disorders. Diarrhea is also treated with an effective astringent medicine that does not stop the flow of the disturbing substance in the stomach; rather, it controls the irritation in the small intestine.
Tannins not only heal burns and stop bleeding, but they also stop infection while they continue to heal the wound internally. The ability of tannins to form a protective layer over the exposed tissue keeps the wound from being infected even more. Tannins are also beneficial when applied to the mucosal lining of the mouth.
Tannins can also be effective in protecting the kidneys. Tannins have been used for immediate relief of sore throats, diarrhea, dysentery, hemorrhaging, fatigue, skin ulcers and as a cicatrizant on gangrenous wounds. Tannins can cause regression of tumors that are already present in tissue, but if used exessively over time, they can cause tumors in healthy tissue. Tannins are used indirectly as molluscicides to interrupt the transmission cycle of schistosomiasis. They have also reported to have anti-viral affects. When incubated with red grape juice and red wines with a high content of condensed tannins, the poliovirus, herpes simplex virus, and various enteric viruses are inactivated.[27]
Tannins can also be used to pull out poisons from poison oak or from bee stings, causing instant relief. The tannins help draw out all irritants from the skin because tannin is an astringent that tightens pores and pulls out liquids.
Tannins produce different colors with ferric chloride (either blue, blue black, or green to greenish black) according to the type of tannin. Iron gall ink is produced by treating a solution of tannins with iron(II) sulfate.
Tannin is a component in a type of industrial particleboard adhesive developed jointly by the Tanzania Industrial Research and Development Organization and Forintek Labs Canada.
Medical potential
Tannins have shown potential antiviral,[28][29][30] antibacterial[31][32] and antiparasitic effects.[33] In the past few years tannins have also been studied for their potential effects against cancer through different mechanisms.[34][35][36]
Tannins, including gallo and ellagic acid (epigallitannins), are inhibitors of HIV replication.
- 1,3,4-Tri-O-galloylquinic acid
- 3,5-di-O-galloyl-shikimic acid
- 3,4,5-tri-O-galloylshikimic acid
- punicalin
- punicalagin
inhibited HIV replication in infected H9 lymphocytes with little cytotoxicity. Two compounds, punicalin and punicacortein C, inhibited purified HIV reverse transcriptase.[37]
References
Notes
- ↑ McGee, Harold. On Food and Cooking. Simon & Schuster. New York, NY 2004 pg714
- ↑ 2.0 2.1 Bate-Smith and Swain, 1962, Flavonoid compounds. In : Comparative biochemistry. Florkin M. Mason H.S. Eds. Vol III. 75-809. Academic Press, New-York.
- ↑ “Chemistry and significance of condensed tannins” (1989). Ed. by Hemingway R.W. and Karchesy J.J.. Plenum Press, New York 47-60.
- ↑ Chavan, J.K. Kadam, S.S. Salunkhe, D.K. Dietary Tannins: Consequences and Remedies. CRC Press 177
- ↑ Hamilton-Miller JM. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob Agents Chemother. 39(11): 2375-2377 (1995) PMID 8585711
- ↑ Wheeler, S.R. Tea and Tannins Science 204: 6-8 (1979) [1] as cited in Yam TS, Hamilton-Miller JM, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2' synthesis, and beta-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 42(2):211-216. (1998) PMID 9738838
- ↑ Hurrell RF, Reddy M, Cook JD. “Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages.” The British journal of nutrition 1999 April; 81 (4): 289-95
- ↑ R. Corder, W. Mullen, N. Q. Khan, S. C. Marks, E. G. Wood, M. J. Carrier and A. Crozier Nature 444, 566 (30 November 2006)
- ↑ Biomed. Pharmacother. 2002, 56, 276-82
- ↑ J. Agric. Food Chem. 2003, 51, 3493-3501
- ↑ J Agric Food Chem 2000 48 (10) 4581-89
- ↑ J Nutr Biochemistry 2005 (16) 360-367
- ↑ Vattem, D. A. Ghaedian, R. Shetty, K. Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry Asia Pacific Journal of Clinical Nutrition (2005) 14(2) 120-130 [2]
- ↑ R. Puupponen-Pimiä, L. Nohynek, C. Meier, M. Kähkönen, M. Heinonen, A. Hopia & K.-M. Oksman-Caldentey, Antimicrobial properties of phenolic compounds from berries, Journal of Applied Microbiology 90(4) pp494 (2001) [3]
- ↑ Navia, Jeanette. “Could Tannins Explain Classic Migraine Triggers?” 1988
- ↑ Reed, J.D."Nutritional toxicology of tannins and related polyphenols in forage legumes". J. Anim. Sci. 1995.73:1516-1528
- ↑ Tannin Chemistry, by Anne E. Hagerman
- ↑ Muller-Harvey, I. and McAllan A.B. (1992). Tannins: Their biochemistry and nutritional properties. Adv. Plant Cell Biochem. and Biotechnol. 1: 151-217.
- ↑ Schiavone, A., Guo, K., Tassone, S., Gasco, L., Hernandez, E., Denti, R. and Zoccarato, I. (2007) Effects of a Natural Extract of Chestnut Wood on Digestibility, performance Traits, and Nitrogen Balance of broiler Chicks. Poultry Science 87: 521–527
- ↑ Tobacco E., Borreani, G., Crovetto G. M., Galassi G., Colombo. D., and Cavallarin. L. (2002) Effect of Chestnut Tannin on Fermentation Quality, Proteolysisand Protein Rumen Degradability of Alfalfa Silage. J.Dairy Sci. 89: 4736–4746
- ↑ Mathieu F and Jouany JP (1993). Effect of chestnut tannin on the fermentability of soyabean meal nitrogen in the rumen. Ann Zootech 42, 127
- ↑ González, S., Pabón, M. L. and Carulla, J. (2002) Effects of tannins on in vitro ammonia release and dry matter degradation of soybean meal. Arch. Latinoam. Prod. Anim. 10(2): 97-101
- ↑ 23.0 23.1 Brune, M. Rossander, L. Hallberq, L. “Iron absorption and phenolic compounds: importance of different phenolic structures” European journal of clinical nutrition, 1989 Aug; 43(8): 547-57
- ↑ Afsana, Kaosar. Hara, Hiroshi. Shiga, Kazuki. “Ingestion of an Indigestible Saccarhride, Difructose Anhydride III, Partially Prevents the Tannic Acid-Induced Suppression of Iron Absorption in Rats.” The Journal of Nutrition. 2003 November 133:33553-3560
- ↑ Hurrell RF, Reddy M, Cook JD. “Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages.” The British journal of nutrition 1999 April; 81 (4): 289-95
- ↑ Lewis, W.H.; and M.P.F. Elvin-Lewis (1977). "Plants Affecting Man's Health". Medical Botany (John Wiley & Sons).
- ↑ Bajaj, Yashpal Singh. Biotechnology in Agriculture and Forestry 24. Medicinal and Aromatic Plants V Springer. 1999.
- ↑ Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antiviral Research 55 (2002) 447/455
- ↑ Main Structural and Stereochemical Aspects of the Antiherpetic Activity of Nonahydroxyterphenoyl-Containing C-Glycosidic Ellagitannins Ste¬phane Quideau, Tatiana Varadinova, Diana Karagiozovac, Michael Jourdesb, Patrick Pardonb), Christian Baudryb, Petia Genovac, Theodore Diakovc, and Rozalina Petrovac Chemistry and biodiversity 1 (2004)
- ↑ Tannin inhibits HIV-1 entry by targeting gp41 . Lin, LU., Shu-wen, L., Shi-bo J., Shu-guang W. Acta Pharmacol Sin 2004 Feb; 25(2): 213-218
- ↑ Antibacterial action of several tannins against staphylococcus aureus. Journal of antimicrobial chemotherapy (2001) 48, 487-491.Akiyama, H., Kazuyasu, F., Yamasaki, O., Oono, T., Iwatsuki, K.
- ↑ Antibacterial activity of hydrolysable tannins derived from medicinal plants against Helicobacter pylori. Funatogawa, K., Hayashi, S., Shimomura, H., Yoshida, T., Hatano, T., Ito, H and Iría, Y Microbiol. Immunol, 48(4): 251-261 (2004)
- ↑ Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells Phytochemistry 66 (2005) 2056–2071
- ↑ p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Bhagavathi A. Narayanana, Otto Geoffroya, Mark C. Willinghama, Gian G. Reb, Daniel W. Nixona. Cancer Letters 136 (1999) 215-221
- ↑ Induction of apoptosis by hydrolyzable tannins from Eugenia jambos L. on human leukemia cells Ling-Ling Yang, Chih-Ying Lee, Kun-Ying Yen Cancer Letters 157 (2000) 65±75
- ↑ Suppression of tumor cell invasiveness by hydrolyzable tannins (plant polyphenols) via the inhibition of matrix metalloproteinase-2/-9 activity Susumu Tanimura a, Ryoji Kadomoto a,b, Takashi Tanaka b, Ying-Jun Zhang b, Isao Kouno b, Michiaki Kohno. Biochemical and Biophysical Research Communications 330 (2005) 1306–1313
- ↑ Anti-AIDS agents, 2: Inhibitory effects of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells
General References
- L. Calvi, G.C.J. Mwalongo, B.A. Mwingira, B. Riedl and J.A. Shields; Characterization of Wattle-Tannin-Based Adhesives for Tanzania (A paper published in Holzforchung Vol 49 No 2, 1995).
External links
|
||||||||||||||