Springtail
| Springtails Fossil range: Early Devonian - Recent |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
 Isotoma sp.
|
||||||||||
| Scientific classification | ||||||||||
|
||||||||||
| Orders | ||||||||||
|
Entomobryomorpha |
||||||||||
| Synonyms | ||||||||||
|
Arthropleona |
Springtails (Collembola) form the largest of the three lineages of modern hexapods that are no longer considered insects (the other two are the Protura and Diplura). The three orders are sometimes grouped together in a class called Entognatha because they have internal mouthparts, but they do not appear to be more closely related to one another than to insects, which have external mouthparts.
DNA sequence studies suggest that Collembola are a separate evolutionary line from the other Hexapoda.[1] Consequently their taxonomic rank has changed according to need: when they were included with the insects, they were ranked as an order; as part of the Entognatha, they are ranked as a subclass. If they are considered a basal lineage of Hexapoda, they are elevated to full class status.
Contents |
Description
Members of Collembola are normally less than 6 mm long, have six or fewer abdominal segments and possess a tubular appendage (the collophore or ventral tube) with eversible vesicles, projecting ventrally from the first abdominal segment. Most species have an abdominal, tail-like appendage, the furcula, that is folded beneath the body to be used for jumping when the animal is threatened. It is held under tension by a small structure called the retinaculum and when released, snaps against the substrate, flinging the springtail into the air.
The Poduromorpha and Entomobryomorpha have an elongated body, while the Symphypleona have a globular body.
Systematics and evolution
Traditionally, the springtails were divided into the orders Arthropleona, Symphypleona and occasionally also Neelipleona. The Arthropleona were divided into two superfamilies, the Entomobryoidea and the Poduroidea. But actually, these two and the Symphypleona form three lineages, each of which is equally distant from the other two. Thus, the "Arthropleona" are abolished in modern classifications, and its superfamilies are raised in rank accordingly, being now the Entomobryomorpha and the Poduromorpha. Technically, the Arthropleona are thus a partial junior synonym of the Collembola. The term "Neopleona" is essentially synonymous with Symphypleona + Neelipleona.
The Neelipleona, which are monotypic, were originally seen as particular advanced lineage of Symphypleona, based on the shared global body shape. But the global body of Neelipleona is realised in a completely different way than in Symphypleona. Currently, the Neelipleona are considered as being derived from the Entomobryomorpha.
Springtails are attested to since the Early Devonian. The fossil from 400 million years ago, Rhyniella praecursor, is the 'oldest' terrestrial arthropod found in the famous Rhynie chert of Scotland. Given its morphology resembles current species quite well, the radiation of the Hexapoda can be situated in the Silurian, 420 million years ago or more.[2]
Ecology
Springtails are cryptozoa frequently found in leaf litter and other decaying material,[3] where they are primarily detritivores and microbivores, and one of the main biological agents responsible for the control and the dissemination of microorganisms.[4]
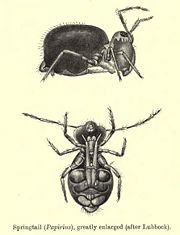
In sheer numbers, they are reputed to be one of the most abundant of all macroscopic animals, with estimates of 100,000 individuals per cubic meter of topsoil, essentially everywhere on Earth where soil and related habitats (moss cushions, fallen wood, grass tufts, ant nests) occur; only nematodes, crustaceans, and mites are likely to have global populations of similar magnitude, and each of those groups except mites is more inclusive: though taxonomic rank cannot be used for absolute comparisons, it is notable that nematodes are a phylum and crustaceans a subphylum. Most springtails are small and difficult to see by casual observation, but one springtail, the so-called snow flea (such as Hypogastrura nivicola), is readily observed on warm winter days when it is active and its dark color contrasts sharply with a background of snow.
In addition, a few species routinely climb trees and form a dominant component of canopy faunas, where they may be collected by beating or insecticide fogging. These tend to be the larger (>2mm) species, mainly in the genera Entomobrya, Orchesella and Lepidocyrtus, though the densities on a per square metre basis are typically 1-2 orders of magnitude lower than soil populations of the same species. A very few species (e.g. Anurophorus spp., Entomobrya albocincta) are almost exclusively arboreal.
The main ecological factor driving locally the distribution of species is the vertical stratification of the environment: in woodlands a continuous change in species assemblages can be observed from tree canopies to ground vegetation then to plant litter down to deeper soil horizons.[5] This a complex factor embracing both nutritional and physiological requirements, together with probable species interactions. Some species have been shown to exhibit negative[6] or positive[7] gravitropism, which adds a behavioural dimension to this still poorly understood vertical segregation.
As a group, springtails are highly sensitive to desiccation, because of their tegumentary respiration: they lack trachea (exceptions exist, such as in Sminthuridae), which forces them to respire through a porous cuticle. The gregarious behaviour of Collembola, mostly driven by the attractive power of pheromones excreted by adults,[8] gives more chance to every juvenile or adult individual to find suitable, better protected places, where desiccation could be avoided and reproduction rate could be kept at an optimum. Sensitivity to dryness varies from species to species and increases during ecdysis. Given that springtails are moulting repeatedly during their entire life (an ancestral character in Hexapoda) they spend much time in concealed micro-sites where they can find protection against desiccation and predation.
The horizontal distribution of springtail species is affected by environmental factors which act at the landscape scale, such as soil acidity, moisture and light.[9] Requirements for pH can be reconstructed experimentally.[10] Altitudinal changes in species distribution can be at least partly explained by increased acidity at higher elevation.[11] Moisture requirements explain why some species cannot live aboveground (vertical stratification), but also why some epigeal springtails are always found in the vicinity of ponds and lakes, such as the hygrophilous Isotomurus palustris. Adaptive features, such as the presence of a fan-like wettable mucro, allow some species to move at the surface of water (Sminthurides aquaticus, Sminthurides malmgreni). Podura aquatica, a unique representative of the family Poduridae (and one of the first springtails to have been described by Linnaeus), spends its entire life at the surface of water, its wettable eggs dropping in water until the non-wettable first instar hatches then surfaces.
In a variegated landscape, made of a patchwork of closed (woodland) and open (meadows, cereal crops) environments, most soil-dwelling species are not specialized and can be found everywhere, but most epigeal and litter-dwelling species are attracted to a particular environment, either forested of not. As a consequence of dispersal limitation, landuse change, when too rapid, may cause the local disappearance of slow-moving, specialized species.[12]
Reproduction
Sexual reproduction occurs through the clustered or scattered deposition of spermatophores by male adults. Stimulation of spermatophore deposition by female pheromones has been demonstrated in Sinella curviseta.[13] Mating behaviour can be observed in Symphypleona.[14] Among Symphypleona, males of Sminthurididae use a clasping organ located on their antenna. Many collembolan species, mostly those living in deeper soil horizons, are parthenogenetic, which favours reproduction to the detriment of genetic diversity and thereby to population tolerance of environmental hazards. Parthenogenesis (also called thelytoky) is under the control of symbiotic bacteria of the genus Wolbachia, which live, reproduce and are carried in female reproductive organs and eggs of Collembola.[15] Feminizing Wolbachia species are widespread in arthropods[16] and nematodes[17] where they co-evolved with most of their lineages.
Relationship with humans
Various sources and publications have suggested that some springtails may parasitize humans, but this is entirely inconsistent with their biology, and no such phenomenon has ever been scientifically confirmed, though it has been documented that the scales or hairs from collembolans can cause irritation when rubbed onto the skin.[18] They can sometimes be abundant indoors in damp places such as bathrooms and basements, and under such circumstances may be found on one's person, but this is only accidental. Claims of persistent human skin infection by springtails may indicate a neurological problem, or else delusory parasitosis, a psychological rather than entomological problem. Researchers themselves may be subject to psychological phenomena. For example, a publication in 2004 claiming that springtails had been found in skin samples was later determined to be a case of pareidolia; that is, no springtail specimens were actually recovered, but the researchers had digitally enhanced photos of sample debris to create images resembling small arthropod heads, which they then claimed to be springtail remnants.[19][18] However, Hopkin reports one instance of an entomologist aspirating an Isotoma species and in the process accidentally inhaling some of their eggs, which hatched in his nasal cavity and made him quite ill until they were flushed out.[3]
Pests or beneficial organisms?
More serious and better documented than parasitoses on humans are the records of springtails as pests for some agricultural crops. Sminthurus viridis, the 'lucerne flea', has been shown to cause severe damage to agricultural crops[20] and is considered as a pest in Australia.[21][22] Also Onychiuridae are known to feed on tubers and to damage them to some extent.[23] However, by their capacity to carry spores of mycorrhizal fungi and mycorrhiza-helper bacteria on their tegument, soil springtails play a positive role in the establishment of plant-fungal symbioses and thus are beneficial to agriculture.[24] They also contribute to control plant fungal diseases through their active consumption of mycelia and spores of damping-off and pathogenic fungi.[25][26] It has been suggested that they could be reared to be used for the control of pathogenic fungi in greenhouses and other indoor cultures.[27][28]
Ecotoxicology
Springtails are currently used in laboratory tests for the early detection of soil pollution. Acute and chronic toxicity tests have been performed by researchers, mostly using the parthenogenetic isotomid Folsomia candida.[29] These tests have been standardized.[30] More recently, avoidance tests have been also performed.[31] They are in way to be standardized, too. Avoidance tests are complementary to toxicity tests, but they also offer several advantages: they are more rapid (thus cheaper), more sensitive and they are environmentally more reliable, because in the real world Collembola may move far from pollution sources.[32] It may be hypothesized that the soil could become locally depauperated in animals (and thus improper to normal use) while below thresholds of toxicity. Contrary to earthworms, and like many insects and molluscs, Collembola are very sensitive to herbicides and thus are threatened in no-tillage agriculture, which makes a more intense use of herbicides than conventional agriculture.
Footnotes
- ↑ Delsuc et al. (2003), Nardi et al. (2003a,b), Hassanin (2006)
- ↑ See references in Haaramo (2008)
- ↑ 3.0 3.1 Hopkin (1997)
- ↑ Ponge (1991)
- ↑ Ponge (1993)
- ↑ Bowden et al. (1976)
- ↑ Didden (1987)
- ↑ Verhoef (1984)
- ↑ Ponge (1993)
- ↑ Van Straalen & Verhoef (1997)
- ↑ Loranger et al. (2001)
- ↑ Ponge et al. (2006)
- ↑ Waldorf (1974)
- ↑ Kozlowski & Aoxiang (2006)
- ↑ Czarnetzki & Tebbe (2004)
- ↑ Werren et al. (1995)
- ↑ Fenn & Blaxter (2004)
- ↑ 18.0 18.1 Janssens & Christiansen (2007)
- ↑ Berenbaum (2005)
- ↑ Shaw & Haughs (1983)
- ↑ Bishop et al. (2001)
- ↑ "PestWeb - Lucerne Flea". http://www.agric.wa.gov.au/ Western Australia Department of Agriculture and Food. Retrieved on 2008-10-11.
- ↑ Baker & Dunning (1975)
- ↑ Klironomos & Moutoglis (1999)
- ↑ Shiraishi et al. (2003)
- ↑ Sabatini & Innocenti (2001)
- ↑ Ponge & Charpentié (1981)
- ↑ Lartey et al. (1989)
- ↑ Fountain & Hopkin (2001)
- ↑ ISO 11267
- ↑ Lors et al. (2006)
- ↑ Chauvat & Ponge (2002)
References
- Baker, A. & Dunning, R. (1975): Association of populations of onychiurid Collembola with damage to sugar-beet seedlings. Plant Pathology 24: 150-154.
- Berenbaum, M. (2005): Face Time. American Entomologist 51: 68-69.
- Bishop A.L., Harris A.M. & McKenzie H.J.. (2001): Distribution and ecology of the lucerne flea, Sminthurus viridis (L.) (Collembola: Sminthuridae), in irrigated lucerne in the Hunter dairying region of New South Wales. Australian Journal of Entomology 40(1):49-55. doi:10.1046/j.1440-6055.2001.00202.x HTML abstract
- Bowden, J.; Haines, I.H. & Mercer, D. (1976): Climbing Collembola. Pedobiologia 16: 298–312.
- Chauvat, M.. & Ponge, J.F. (2002): Colonization of heavy metal-polluted soils by collembola: preliminary experiments in compartmented boxes. Applied Soil Ecology 21(2): 91-106. doi:10.1016/S0929-1393(02)00087-2 (HTML abstract)
- Czarnetzki, A.B. & Tebbe, C.C. (2004): Detection and phylogenetic analysis of Wolbachia in Collembola. Environmental Microbiology 6: 35-44. doi:10.1046/j.1462-2920.2003.00537.x HTML abstract
- Delsuc, Frédéric; Phillips, Matthew J. & Penny, David (2003): Comment on "Hexapod Origins: Monophyletic or Paraphyletic?" Science 301(5639): 1482. doi:10.1126/science.1086558 PDF fulltext
- Didden, W.A.M. (1987): Reactions of Onychiurus fimatus (Collembola) to loose and compact soil methods and first results. Pedobiologia 30(2): 93-100. HTML abstract
- Fenn, K. & Blaxter, M. (2004): Are filarial nematode Wolbachia obligate mutualist symbionts? Trends Ecol. Evol. 19(4): 163-166. doi:10.1016/j.tree.2004.01.002 (HTML abstract)
- Fountain, M.T. & Hopkin, S.P. (2001): Continuous monitoring of Folsomia candida (Insecta: Collembola) in a metal exposure test. Ecotoxicology and Environmental Safety 48(3): 275-286. doi:10.1006/eesa.2000.2007 (HTML abstract)
- Haaramo, Mikko (2008): Mikko's Phylogeny Archive - Collembola. Version of 2008-MAR-11. Retrieved 2008-JUL-11.
- Hassanin, Alexandre (2006): Phylogeny of Arthropoda inferred from mitochondrial sequences: Strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution. Mol. Phylogenet. Evol. 38(1): 100-116. doi:10.1016/j.ympev.2005.09.012 (HTML abstract)
- Hopkin, Stephen P. (1997): The Biology of the Collembola (Springtails): The Most Abundant Insects in the World. Natural History Museum, London. PDF fulltext
- ISO 11267 (1999): Soil quality. Inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. International Organization for Standardization. [1]
- Janssens, Frans & Christiansen, Kenneth A. (2007): Checklist of the Collembola - Synanthropic Collembola, Springtails in Association with Man. Version of 2007-NOV-22. Retrieved 2008-JUL-11.
- Klironomos, J.N. & Moutoglis, P. (1999): Colonization of nonmycorrhizal plants by mycorrhizal neighbours as influenced by the collembolan, Folsomia candida. Biology and Fertility of Soils 29(3): 277-281. doi:10.1007/s003740050553 (HTML abstract)
- Kozlowski, M.W. & Aoxiang, S. (2006): Ritual behaviors associated with spermatophore transfer in Deuterosminthurus bicinctus (Collembola : Bourletiellidae). Journal of Ethology 24(2): 103-110. doi:10.1007/s10164-005-0162-6 (HTML abstract)
- Lartey, R.T., Curl, E.A., Peterson, C.M. & Harper, J.D. (2006): Ritual behaviors associated with spermatophore transfer in Deuterosminthurus bicinctus (Collembola : Bourletiellidae). Journal of Ethology 24(2): 103-110. doi:10.1007/s10164-005-0162-6 (HTML abstract)
- Loranger, G., Bandyopadhyaya, I., Razaka, B. & Ponge, J.F. (1989): Does soil acidity explain altitudinal sequences in collembolan communities?. Soil Biology and Biochemistry 33(3): 381–393. doi:10.1016/S0038-0717(00)00153-X (HTML abstract)
- Lors, C., Martinez Aldaya, M., Salmon, S. & Ponge, J.F. (2006): Use of an avoidance test for the assessment of microbial degradation of PAHs. Soil Biology and Biochemistry 38(8): 2199-2204. doi:10.1016/j.soilbio.2006.01.026 (HTML abstract)
- Nardi, Francesco; Spinsanti, Giacomo; Boore, Jeffrey L.; Carapelli, Antonio; Dallai, Romano & Frati, Francesco (2003a): Hexapod Origins: Monophyletic or Paraphyletic? Science 299(5614): 1887-1889. doi:10.1126/science.1078607 Supporting Online Material
- Nardi, Francesco; Spinsanti, Giacomo; Boore, Jeffrey L.; Carapelli, Antonio; Dallai, Romano & Frati, Francesco (2003b): Response to Comment on "Hexapod Origins: Monophyletic or Paraphyletic?" Science 301(5639): 1482. doi:10.1126/science.1087632 PDF fulltext
- Ponge, J.F. (1991): Food resources and diets of soil animals in a small area of Scots pine litter. Geoderma 49(1-2): 33–62. doi:10.1016/0016-7061(91)90090-G (HTML abstract)
- Ponge, J.F. (1993): Biocenoses of Collembola in atlantic temperate grass-woodland ecosystems. Pedobiologia 37(4): 223-244.
- Ponge, J.F. & Charpentié, M.J. (1981): Étude des relations microflore-microfaune: expériences sur Pseudosinella alba (Packard), Collembole mycophage. Revue d'Ecologie et de Biologie du Sol 18: 291-303.
- Ponge, J.F., Dubs, F., Gillet, S., Sousa, J.P. & Lavelle, P. (2006): Decreased biodiversity in soil springtail communities: the importance of dispersal and landuse history in heterogeneous landscapes. Soil Biology & Biochemistry 38: 1158-1161. doi:10.1016/j.soilbio.2005.09.004 (HTML abstract)
- Sabatinii, M.A. & Innocenti, G. (2001): Effects of Collembola on plant-pathogenic fungus interactions in simple experimental systems. Biology and Fertility of Soils 33(1): 62-66. doi:10.1007/s003740000290 (HTML abstract)
- Shaw, M. W., Haughs, G. M. (1983): Damage to potato foliage by Sminthurus viridis (L.). Plant Pathology 32(4): 465-466. doi:10.1111/j.1365-3059.1983.tb02864.x
- Shiraishi, H., Enami, Y. & Okano, S. (2003): Folsomia hidakana (Collembola) prevents damping-off disease in cabbage and Chinese cabbage by Rhizoctonia solani. Pedobiologia 47(1): 33-38. doi:10.1078/0031-4056-00167 (HTML abstract)
- Van Straalen, N.M. & Verhoef, H.A. (1997): The development of a bioindicator system for soil acidity based on arthropod pH preferences.Journal of Applied Ecology 34(1): 217–232.
- Verhoef, H.A. (1984): Releaser and primer pheromones in Collembola. Journal of Insect Physiology 30(8): 665-670.
- Waldorf, E.S. (1974): Sex pheromone in the springtail Sinella curviseta. Environmental Entomology 3: 916–918.
- Werren, J.H.; Zhang, W. & Guo, L.R. (1995): Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proceedings of the Royal Society of London, Series B, Biological Sciences 261(1360): 55–63. PMID 7644549 HTML abstract and first page image
External links
- Maps of Collembola (Britain and Ireland) also Photolibrary
- General information on Collembola
- Checklist of the Collembola of the World
- The Biology of the Collembola
- Tree of Life
- The Springtails (Collembola) of South Africa
- North American Collembola
- Springtails, Kansas State University
Specialists
See complete list[2]
- Bengtsson Göran[3]
- Berg Matty[4]
- Chagnon Madeleine[5]
- Chauvat Matthieu[6]
- Christian Erhard[7]
- Christiansen Kenneth A.
- Cortet Jérôme[8]
- Crouau Yves[9]
- Deharveng Louis[10]
- Dunger Wolfram
- Eisenbeis Gerhard[11]
- Filser Juliane[12]
- Fountain Michelle
- Frati Francesco[13]
- Fjellberg Arne[14]
- Frampton Geoffrey[15]
- Gers Charles[16]
- Greenslade Penelope[17]
- Gruia Magdalena
- Hågvar Sigmund[18]
- Hedlund Katarina [19]
- Holmstrup Martin[20]
- Huhta Veikko[21]
- Janssens Frans[22]
- Jordana Rafael[23]
- Kampichler Christian[24]
- Krogh Paul Henning[25]
- Lee Byung Hoon
- Mari Mutt José A.[26]
- Mateos Eduardo[27]
- Najt Judith
- Palacios-Vargas José G.
- Pomorski Romuald Jacek
- Ponge Jean-François[28][29]
- Potapov Mikhail
- Rusek Josef[30]
- Salmon Sandrine
- Shaw Peter[31]
- Simon Benito José Carlos
- Snider Richard J.[32]
- Sousa Jorge Paulo[33]
- Takeda Hiroshi[34]
- Thibaud Jean-Marc
- Van Straalen Nico M.[35]
- Verhoef Herman A.[36]
- Weiner Wanda
- Wolters Volkmar[37]
- Zettel Jürg[38]