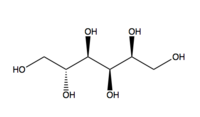
Sorbitol
| Sorbitol | |
|---|---|
 |
|
| IUPAC name | (2S,3R,4R,5R)-Hexane-1,2,3,4,5,6-hexol |
| Identifiers | |
| CAS number | 50-70-4 |
| PubChem | |
| MeSH | |
| SMILES |
|
| Properties | |
| Molecular formula | C6H14O6 |
| Molar mass | 182.17 g/mol |
| Density | 1.489 g/cm³ |
| Melting point |
95 °C |
| Boiling point |
296 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Sorbitol, also known as glucitol, is a sugar alcohol that the body metabolises slowly. It is obtained by reduction of glucose changing the aldehyde group to an additional hydroxyl group hence the name sugar alcohol.
Contents |
Uses
Sweetener
Sorbitol is used in "sugar-free" mints and various cough syrups and is usually listed under the inactive ingredients.
Sorbitol is a sugar substitute often used in diet foods (including diet drinks and ice cream) and sugar-free chewing gum. It also occurs naturally in many stone fruits and berries from trees of the genus Sorbus[1]. Sorbitol is also referred to as a nutritive sweetener because it provides dietary energy: 2.6 kilocalories (11 kilojoules) per gram versus the average 4 kilocalories (17 kilojoules).
Laxative
Sorbitol can be used as a non-stimulant laxative as either an oral suspension or suppository. The drug works by drawing water into the large intestine, thereby stimulating bowel movements.[2] Sorbitol has been determined safe to use in the elderly although it is by no means recommended. [3]
Medical importance
Sorbitol is used in bacterial culture media to distinguish Escherichia_coli_O157_H7 from most other strains of E Coli.
In some human enzymes deficiencies, sorbitol excess arises and can cause damage to the body, although in individuals without certain genetic mutations, it is normal part in the chain of carbohydrate metabolism. An example of such a disease is galactosaemia. In diabetes mellitus, the enzyme is not present in sufficient quantities in some tissue, such as the lens of the eye. Consequently, sorbitol can build up causing cataracts.
Miscellanea
Sorbitol is often used in modern cosmetics as a humectant and thickener. Some transparent gels can only be made with sorbitol as it has a refractive index sufficiently high for transparent formulations. It is also used as a humectant in some cigarettes.[4]
Sorbitol is used as a cryoprotectant additive (mixed with sucrose and sodium polyphosphates) in the manufacture of surimi, a highly refined, uncooked fish paste most commonly produced from Alaska (or walleye) pollock (Theragra chalcogramma).
Sorbitol, combined with kayexalate, helps the body rid itself of excess potassium ions in a hyperkalaemic state. The kayexalate exchanges sodium ions for potassium ions in the bowel, while sorbitol helps to eliminate it.
Sorbitol when combined with potassium nitrate has found some success as an amateur solid rocket fuel.[5]
Sorbitol is often used in mouthwash, as it is said that when mixed with other certain ingredients it can help fight plaque.
Sorbitol is identified as a potential key chemical intermediate [6] from biomass resources. Complete reduction of sorbitol opens the way to alkanes such as hexane which can be used as a biofuel. Sorbitol itself provides much of the hydrogen required for the transformation.
- 19 C6O6H14 → 13 C6H14 + 36 CO2 + 42 H2O
The above chemical reaction is exothermic and 1.5 mole of sorbitol generates 1 mole of hexane. When hydrogen is co-fed, no carbon dioxide production takes place.
Overdose effects
Ingesting large amounts of sorbitol can lead to some abdominal pain, gas, and mild to severe diarrhea. Sorbitol ingestion of 20 grams/day (g/d) as sugar-free gum has led to severe diarrhea leading to unintended weight loss of 24 lbs in a 114 lb woman; another patient required hospitalization after habitually consuming 30g/d.[7] Sorbitol can also aggravate irritable bowel syndrome and fructose malabsorption. [8]
Even in the absence of dietary sorbitol, cells produce sorbitol naturally. When too much sorbitol is produced inside cells, it can cause damage.[9] Diabetic retinopathy and neuropathy may be related to excess sorbitol in the cells of the eyes and nerves. The source of this sorbitol in diabetics is excess glucose, which goes through the polyol pathway [10].
Safety
Sorbitol’s safety is supported by numerous studies reported in the scientific literature. In developing the current U.S. food and drug regulation which affirms sorbitol as GRAS, the safety data were carefully evaluated by qualified scientists of the Select Committee on GRAS Substances selected by the Life Sciences Office of the Federation of American Societies for Experimental Biology (FASEB). In the opinion of the Select Committee, there was no evidence demonstrating a hazard where sorbitol was used at current levels or at levels that might be expected in the future. The U.S. Food and Drug Administration’s regulation for sorbitol requires the following label statement for foods whose reasonably foreseeable consumption may result in the daily ingestion of 50 grams of sorbitol: “Excess consumption may have a laxative effect.”
The Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) has reviewed the safety data and concluded that sorbitol is safe. JECFA has established an acceptable daily intake (ADI) for sorbitol of “not specified,” meaning no limits are placed on its use. An ADI “not specified” is the safest category in which JECFA can place a food ingredient. JECFA’s decisions are often adopted by many small countries which do not have their own agencies to review food additive safety.
The Scientific Committee for Food of the European Union (EU) published a comprehensive assessment of sweeteners in 1985, concluding that sorbitol is acceptable for use, also without setting a limit on its use.
See also
- Mannitol
- Xylitol
External links
- NIH Diabetes dictionary — see entry on sorbitol
References
- ↑ Lehninger Principles of Biochemistry, Nelson and Cox, Fourth Edition
- ↑ ACS :: Cancer Drug Guide: sorbitol
- ↑ Lederle FA: Epidemiology of constipation in elderly patients. Drug utilization and cost-containment strategies. Drugs and Ageing 6:465-469, 1995.
- ↑ Gallaher Group Plc - Ingredients
- ↑ Richard Nakka's Experimental Rocketry Web Site
- ↑ Production of Liquid Hydrocarbons from Biomass Jürgen O. Metzger Angewandte Chemie International Edition Volume 45, Issue 5 , Pages 696 - 698 2005 Abstract
- ↑ Kathleen Doheny (2008-01-10). "Sweetener Side Effects: Case Histories". WebMD Medical News. Retrieved on 2008-01-10.
- ↑ Hope, Jenny (11th January 2008). "Sweetener in chewing gum can damage your health", Daily Mail. Retrieved on 2008-01-11. "A sweetener used in sugar-free chewing gum, some toothpastes and thousands of other products could be a severe health risk, doctors warned." Reports The British Medical Journal.
- ↑ Sorbitol: a hazard for diabetics? Nutrition Health Review
- ↑ Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage.
|
|||||||||||||||||||||||||||||||||||