Vapor pressure
Vapor pressure (also known as equilibrium vapor pressure), is the pressure of a vapor in equilibrium with its non-vapor phases. All liquids and solids have a tendency to evaporate to a gaseous form, and all gases have a tendency to condense back into their original form (either liquid or solid). At any given temperature, for a particular substance, there is a pressure at which the gas of that substance is in dynamic equilibrium with its liquid or solid forms. This is the vapor pressure of that substance at that temperature. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of molecules and atoms to escape from a liquid or a solid. A substance with a high vapor pressure at normal temperatures is often referred to as volatile.
The vapor pressure of any substance increases non-linearly with temperature according to the Clausius-Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature where the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher pressure, and therefore higher temperature, because the fluid pressure increases above the atmospheric pressure as the depth increases.
Contents |
Relation between vapor pressures and normal boiling points of liquids

The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid.
The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids.[1] As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points.
For example, at any given temperature, propane has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point(-42.1 °C), which is where the vapor pressure curve of propane (the purple line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure.
Although the relation between vapor pressure and temperature is non-linear, the chart uses a logarithmic vertical axis in order to obtain slightly curved lines so that one chart can graph many liquids.
Units of vapor pressure
The international SI unit for pressure is the pascal (Pa), equal to one newton per square meter (N·m-2 or kg·m-1·s-2). The conversions to other pressure units are:
pascal (Pa) |
bar (bar) |
technical atmosphere (at) |
atmosphere (atm) |
torr (Torr) |
pound-force per square inch (psi) |
|
|---|---|---|---|---|---|---|
| 1 Pa | ≡ 1 N/m2 | 10−5 | 1.0197×10−5 | 9.8692×10−6 | 7.5006×10−3 | 145.04×10−6 |
| 1 bar | 100,000 | ≡ 106 dyn/cm2 | 1.0197 | 0.98692 | 750.06 | 14.5037744 |
| 1 at | 98,066.5 | 0.980665 | ≡ 1 kgf/cm2 | 0.96784 | 735.56 | 14.223 |
| 1 atm | 101,325 | 1.01325 | 1.0332 | ≡ 1 atm | 760 | 14.696 |
| 1 torr | 133.322 | 1.3332×10−3 | 1.3595×10−3 | 1.3158×10−3 | ≡ 1 Torr; ≈ 1 mmHg | 19.337×10−3 |
| 1 psi | 6,894.76 | 68.948×10−3 | 70.307×10−3 | 68.046×10−3 | 51.715 | ≡ 1 lbf/in2 |
Example reading: 1 Pa = 1 N/m2 = 10−5 bar = 10.197×10−6 at = 9.8692×10−6 atm, etc.
Note: mmHg is an abbreviation for millimetres of mercury.
Vapor pressure of solids
Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a crystal, this can be defined as the pressure when the rate of sublimation of a solid matches the rate of deposition of its vapor phase. For most solids this pressure is very low, but some notable exceptions are naphthalene, dry ice (the vapor pressure of dry ice is 5.73 MPa (831 psi, 56.5 atm) at 20 degrees Celsius, meaning it will cause most sealed containers to explode), and ice. All solid materials have a vapor pressure. However, due to their often extremely low values, measurement can be rather difficult. Typical techniques include the use of thermogravimetry and gas transpiration.
Water vapor pressure

Water, like all liquids, starts to boil when its vapor pressure reaches its surrounding pressure. At higher elevations the atmospheric pressure is lower and water will boil at a lower temperature. The boiling temperature of water for pressures around atmospheric pressure can be approximated by this Antoine equation:
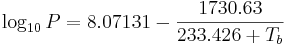
or transformed into this temperature-explicit form:
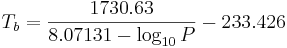
where the temperature 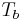 is the boiling point temperature in degrees Celsius and the pressure
is the boiling point temperature in degrees Celsius and the pressure 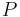 is in Torr.
is in Torr.
Vapor pressure of mixtures
Raoult's law gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity (pressure or fugacity) of a single-phase mixture is equal to the mole-fraction-weighted sum of the components' vapor pressures:
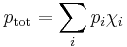
where p is vapor pressure, i is a component index, and χ is a mole fraction. The term 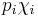 is the vapor pressure of component i in the mixture. Raoult's Law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
is the vapor pressure of component i in the mixture. Raoult's Law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being "held in" the liquid phase less strongly than in the pure liquid. An example is the azeotrope of approximately 95% ethanol and water. Because the azeotrope's vapor pressure is higher than predicted by Raoult's law, it boils at a temperature below that of either pure component.
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are "held in" the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane (chloroform) and 2-propanone (acetone), which boils above the boiling point of either pure component.
Usage of the term vapor pressure in meteorology
In meteorology, the term vapor pressure is used to mean the partial pressure of water vapor in the atmosphere, even if it is not equilibrium,[2] and the equilibrium vapor pressure is specified as such. Meteorologists also use the term saturation vapor pressure to refer to the equilibrium vapor pressure of water or brine above a flat surface, to distinguish it from equilibrium vapor pressure which takes into account the shape and size of water droplets and particulates in the atmosphere.[3]
See also
- Absolute humidity
- Clausius-Clapeyron Equation
- Partial pressure
- Relative humidity
- Relative volatility
- Triple point
- Vapor-liquid equilibrium
- Vapor Pressure of Water at Various Temperatures
- Volatility
- Antoine equation
References
- ↑ Perry, R.H. and Green, D.W. (Editors) (1997). Perry's Chemical Engineers' Handbook (7th Edition ed.). McGraw-Hill. ISBN 0-07-049841-5.
- ↑ Glossary (Developed by the American Meteorological Society)
- ↑ A Brief Tutorial (An article about the definition of equilibrium vapor pressure)