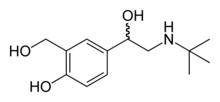
Salbutamol
 |
|
 |
|
|
Salbutamol
|
|
| Systematic (IUPAC) name | |
| 4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol | |
| Identifiers | |
| CAS number | |
| ATC code | R03 R03 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C13H21NO3 |
| Mol. mass | 239.311 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Half life | 1.6 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral, inhalational, IV |
Salbutamol (INN) or albuterol (USAN) is a short-acting β2-adrenergic receptor agonist used for the relief of bronchospasm in conditions such as asthma and chronic obstructive pulmonary disease. It is marketed by GlaxoSmithKline as Ventolin or Ventorlin depending on the market; by Cipla as Asthalin; by Schering-Plough as Proventil and by Teva as ProAir. Many other generic trade names also exist.
Salbutamol sulfate is usually given by the inhaled route for direct effect on bronchial smooth muscle. This is usually achieved through a metered dose inhaler (MDI), nebuliser or other proprietary delivery devices (e.g. Rotahaler or Autohaler). In these forms of delivery, the maximal effect of Salbutamol can take place within five to twenty minutes of dosing, though some relief is immediately seen. Salbutamol can also be given orally as an inhalant or intravenously. However, some asthmatics do not have the required DNA base sequence in a specific gene and may not respond to these medications.
Salbutamol became available in the United Kingdom in 1969 and in the United States in 1980 under the trade name Ventolin.
Contents |
Clinical use
Salbutamol is specifically indicated in the following conditions:
- Acute asthma
- Symptom relief during maintenance therapy of asthma and other conditions with reversible airways obstruction (including COPD and bronchitis)
- Protection against exercise-induced asthma
- Can induce Hypokalemia, especially in patients with renal failure
- Can be aerosolized with a nebulizer for patients with cystic fibrosis, along with ipratropium bromide and pulmozyme.
As a β2-agonist, salbutamol also finds use in obstetrics. Intravenous salbutamol can be used as a tocolytic to relax the uterine smooth muscle to delay premature labour. While preferred over agents such as atosiban and ritodrine, its role has largely been replaced by the calcium-channel blocker nifedipine which is more effective, better tolerated and orally administered.[1]
Side Effects / Health Consequences
- Tachycardia (rapid heart rate)
- Shakiness, nervousness
- Hyperkinesis
- Nausea and Vomiting
- Reactive bronchospasm
- Drowsiness
- Aggression
- Agitation
- Allergic reaction
- Anxiety
- Back Pain
- Excitement
- Fluid Retention and Swelling
- Flushing
- General Bodily Discomfort
- Headache
- Heart Palpitations
- Heartburn
- Hives
- Hyperactivity
- Hypertension
- Increased Appetite
- Increased Difficulty Breathing
- Indigestion
- Insomnia, especially in children
- Irritability
- Lightheadedness
- Muscle Cramps
- Muscle tremor
- Nasal Inflammation
- Nervousness
- Nightmares
- Nosebleed
- Rash
- Throat irritation
- Tooth Discoloration
- Tremors
- Unusual Taste
- Urinary Problems
- Weakness
- Wheezing
- Xerostomia (dry mouth)
Ban of CFC-containing salbutamol inhalers

The U.S. Food & Drug Administration in April 2005 mandated that all (including salbutamol) inhalers containing chlorofluorocarbons (CFCs) will be prohibited in the United States as of 12/31/2008. CFC inhalers had previously been given "essential use" status, exempting it from a CFC-production ban, however in accordance with the Montreal Protocol they will be phased out; in many other countries patients have been transitioned to non-CFC based inhalers using hydrofluoroalkane (HFA) propellant. Pharmaceutical manufacturers are expected to produce adequate supplies of alternative (HFA) inhalers by 2009.
One drawback of this transition to HFA inhalers is that due to patent restrictions all of the HFA salbutamol inhalers are "brand-name" (ProAir, Proventil, and Ventolin). They cost approximately $20 more per inhaler than existing generic CFC salbutamol inhalers.
Generic HFA salbutamol inhalers are not expected on the United States market until 2017 due to existing patents.
Another drawback that is coming to light now as the use of HFA/HFA+ethanol inhalers is expanding seems to be a far higher intolerance of the new inhalers compared to CFC propellant in patients. Registered complaints run the gamut from "doesn't seem to work as well" all the way to serious anaphylaxis in response to using an HFA or HFA+ethanol inhaler.[2]
Salbutamol is widely used, and accounts for anywhere from 78% of all bronchodilator prescriptions in 2005 to 85% in 2008. [3] However, patients in the United States who cannot tolerate the HFA salbutamol inhalers will not have a single salbutamol alternative available to them domestically after December 31, 2008.[4] The FDA did not approve any alternatives to HFA and there are few standard inhaled lung medications in the United States that come in Dry Powder Inhaler (DPI) versions. Noticeably missing is salbutamol in DPI form in the United States, although it is available in most of the rest of the world in salbutamol DPIs.
Diet and bodybuilding use
Salbutamol is taken by some as an alternative to Clenbuterol for purposes of fat burning.[5]
References
- ↑ Rossi S (Ed.) (2004). Australian Medicines Handbook 2004 (AMH). Adelaide: Australian Medicines Handbook. ISBN 0-9578521-4-2.
- ↑ Home Page
- ↑ IMS Health Sales & Prescription data for all inhalers sales and prescriptions (July 2008)
- ↑ FDA Advises Patients to Switch to HFA-Propelled Albuterol Inhalers Now
- ↑ Carter WJ, Lynch ME (September 1994). "Comparison of the effects of salbutamol and clenbuterol on skeletal muscle mass and carcass composition in senescent rats". Metab. Clin. Exp. 43 (9): 1119–25. PMID 7916118. http://linkinghub.elsevier.com/retrieve/pii/0026-0495(94)90054-X.
Additional notes
- Moore NG, Pegg GG, Sillence MN (September 1994). "Anabolic effects of the beta 2-adrenoceptor agonist salmeterol are dependent on route of administration". Am. J. Physiol. 267 (3 Pt 1): E475–84. PMID 7943228. http://ajpendo.physiology.org/cgi/pmidlookup?view=reprint&pmid=7943228.
- Schiffelers SL, Saris WH, Boomsma F, van Baak MA (May 2001). "beta(1)- and beta(2)-Adrenoceptor-mediated thermogenesis and lipid utilization in obese and lean men". J. Clin. Endocrinol. Metab. 86 (5): 2191–9. PMID 11344226. http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=11344226.
- van Baak MA, Mayer LH, Kempinski RE, Hartgens F (July 2000). "Effect of salbutamol on muscle strength and endurance performance in nonasthmatic men". Med Sci Sports Exerc 32 (7): 1300–6. PMID 10912897. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0195-9131&volume=32&issue=7&spage=1300.
- Caruso JF, Hamill JL, De Garmo N (February 2005). "Oral albuterol dosing during the latter stages of a resistance exercise program". J Strength Cond Res 19 (1): 102–7. doi:. PMID 15705021.
- Caruso JF, Signorile JF, Perry AC, et al (November 1995). "The effects of albuterol and isokinetic exercise on the quadriceps muscle group". Med Sci Sports Exerc 27 (11): 1471–6. PMID 8587482.
- Martineau L, Horan MA, Rothwell NJ, Little RA (November 1992). "Salbutamol, a beta 2-adrenoceptor agonist, increases skeletal muscle strength in young men". Clin. Sci. 83 (5): 615–21. PMID 1335400.S
- Desaphy JF, Pierno S, De Luca A, Didonna P, Camerino DC (March 2003). "Different ability of clenbuterol and salbutamol to block sodium channels predicts their therapeutic use in muscle excitability disorders". Mol. Pharmacol. 63 (3): 659–70. PMID 12606775. http://molpharm.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12606775.
- Maki KC, Skorodin MS, Jessen JH, Laghi F (June 1996). "Effects of oral albuterol on serum lipids and carbohydrate metabolism in healthy men". Metab. Clin. Exp. 45 (6): 712–7. PMID 8637445.
External links
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||