International System of Units
The International System of Units (abbreviated SI from the French Le Système International d'Unités[1]) is the modern form of the metric system and is generally a system devised around the convenience of the number ten. It is the world's most widely used system of measurement, both in everyday commerce and in science.[2][3]
The older metric system included several groups of units. The SI was developed in 1960 from the old metre-kilogram-second (mks) system, rather than the centimetre-gram-second (cgs) system, which, in turn, had a few variants. Because the SI is not static, units are created and definitions are modified through international agreement among many nations as the technology of measurement progresses, and as the precision of measurements improves.
The system is nearly universally employed, and most countries do not even maintain official definitions of any other units. A notable exception is the United States, which continues to use customary units in addition to SI. In the United Kingdom, conversion to metric units is government policy, but the transition is not quite complete. Those countries that still recognise non-SI units (e.g., the US and UK) have redefined their traditional non-SI units in SI units.

Contents |
Realisation of units
It is important to distinguish between the definition of a unit and its realisation. The definition of each base unit of the SI is carefully drawn up so that it is unique and provides a sound theoretical basis upon which the most accurate and reproducible measurements can be made. The realisation of the definition of a unit is the procedure by which the definition may be used to establish the value and associated uncertainty of a quantity of the same kind as the unit. A description of how the definitions of some important units are realised in practice is given on the BIPM website.[4]
A coherent SI derived unit can be expressed in SI base units with no numerical factor other than the number 1.[5] The coherent SI derived unit of resistance, the ohm, symbol Ω, for example, is uniquely defined by the relation Ω = m2·kg·s−3·A−2, which follows from the definition of the quantity electrical resistance. However, any method consistent with the laws of physics could be used to realise any SI unit.[6]
History
The metric system was conceived by a group of scientists (among them, Antoine-Laurent Lavoisier, who is known as the "father of modern chemistry") who had been commissioned by Louis XVI of France to create a unified and rational system of measures. After the French Revolution, the system was adopted by the new government.[7] On August 1, 1793, the National Convention adopted the new decimal "metre" with a provisional length as well as the other decimal units with preliminary definitions and terms. On April 7, 1795 (Loi du 18 germinal, an III) the terms "gramme" and "kilogramme" replaced the former terms "gravet" (correctly "milligrave") and "grave". On December 10, 1799 (a month after Napoleon's coup d'etat), the metric system was definitively adopted in France.
The history of the metric system has seen a number of variations, whose use has spread around the world, to replace many traditional measurement systems. At the end of World War II a number of different systems of measurement were still in use throughout the world. Some of these systems were metric-system variations, whereas others were based on customary systems. It was recognised that additional steps were needed to promote a worldwide measurement system. As a result the 9th General Conference on Weights and Measures (CGPM), in 1948, asked the International Committee for Weights and Measures (CIPM) to conduct an international study of the measurement needs of the scientific, technical, and educational communities.
Based on the findings of this study, the 10th CGPM in 1954 decided that an international system should be derived from six base units to provide for the measurement of temperature and optical radiation in addition to mechanical and electromagnetic quantities. The six base units that were recommended are the metre, kilogram, second, ampere, degree Kelvin (later renamed the kelvin), and the candela. In 1960, the 11th CGPM named the system the International System of Units, abbreviated SI from the French name: Le Système international d'unités. The seventh base unit, the mole, was added in 1971 by the 14th CGPM.
Future development
ISO 31 contains recommendations for the use of the International System of Units; for electrical applications, in addition, IEC 60027 has to be taken into account. As of 2008, work is proceeding to integrate both standards into a joint standard Quantities and Units in which the quantities and equations used with SI are to be referred as the International System of Quantities (ISQ).[8]
Units
The international system of units consists of a set of units together with a set of prefixes. The units of SI can be divided into two subsets. There are seven base units: Each of these base units represents, at least in principle, different kinds of physical quantities. From these seven base units, several other units are derived. In addition to the SI units, there is also a set of non-SI units accepted for use with SI.
| Name | Symbol | Quantity |
|---|---|---|
| metre | m | length |
| kilogram | kg | mass |
| second | s | time |
| ampere | A | electric current |
| kelvin | K | thermodynamic temperature |
| candela | cd | luminous intensity |
| mole | mol | amount of substance |
A prefix may be added to a unit to produce a multiple of the original unit. All multiples are integer powers of ten. For example, kilo- denotes a multiple of a thousand and milli- denotes a multiple of a thousandth; hence there are one thousand millimetres to the metre and one thousand metres to the kilometre. The prefixes are never combined: a millionth of a kilogram is a milligram not a microkilogram.
| Multiples | Name | deca- | hecto- | kilo- | mega- | giga- | tera- | peta- | exa- | zetta- | yotta- | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symbol | da | h | k | M | G | T | P | E | Z | Y | ||
| Factor | 100 | 101 | 102 | 103 | 106 | 109 | 1012 | 1015 | 1018 | 1021 | 1024 | |
| Subdivisions | Name | deci- | centi- | milli- | micro- | nano- | pico- | femto- | atto- | zepto- | yocto- | |
| Symbol | d | c | m | µ | n | p | f | a | z | y | ||
| Factor | 100 | 10−1 | 10−2 | 10−3 | 10−6 | 10−9 | 10−12 | 10−15 | 10−18 | 10−21 | 10−24 | |
SI writing style
- Symbols do not have an appended period/full stop (.).
- Symbols are written in upright (Roman) type (m for metres, l for litres), so as to differentiate from the italic type used for variables (m for mass, l for length). By consensus of international standards bodies, this rule is applied independent of the font used for surrounding text.[10]
- Symbols for units are written in lower case, except for symbols derived from the name of a person. For example, the unit of pressure is named after Blaise Pascal, so its symbol is written "Pa", whereas the unit itself is written "pascal". All symbols of prefixes larger than 103 (kilo) are also uppercase.
- The one exception is the litre, whose original symbol "l" is unsuitably similar to the numeral "1" or the uppercase letter "i" (depending on the typeface used), at least in many English-speaking countries. The American National Institute of Standards and Technology recommends that "L" be used instead, a usage which is common in the US, Canada, Australia (but not elsewhere). This has been accepted as an alternative by the CGPM since 1979. The cursive ℓ is occasionally seen, especially in Japan and Greece, but this is not currently recommended by any standards body. For more information, see Litre.
- The SI rule is that symbols of units are not pluralised, for example "25 kg" (not "25 kgs").[10]
- The American National Institute of Standards and Technology has defined guidelines for American users of the SI.[11][12] These guidelines give guidance on pluralising unit names: the plural is formed by using normal English grammar rules, for example, "henries" is the plural of "henry". The units lux, hertz, and siemens are exceptions from this rule: They remain the same in singular and plural. Note that this rule applies only to the full names of units, not to their symbols.
- A space separates the number and the symbol; e.g., "2.21 kg", "7.3×102 m2", "22 K".[13][14] This rule explicitly includes the percent sign. Exceptions are the symbols for plane angular degrees, minutes and seconds (°, ′ and ″), which are placed immediately after the number with no intervening space.
- Spaces may be used as a thousands separator (1 000 000) in contrast to commas or periods (1,000,000 or 1.000.000) in order to reduce confusion resulting from the variation between these forms in different countries. In print, the space used for this purpose is typically narrower than that between words (commonly a thin space).
- Any line-break inside a number, inside a compound unit or between number and unit should be avoided, but, if necessary, the latter option should be used.
- The 10th resolution of CGPM in 2003 declared that "the symbol for the decimal marker shall be either the point on the line or the comma on the line." In practice, the decimal point is used in English and the comma in most other European languages.
- Symbols for derived units formed from multiple units by multiplication are joined with a space or centre dot (·), for example "N m" or "N·m".[15]
- Symbols formed by division of two units are joined with a solidus (⁄), or given as a negative exponent. For example, the "metre per second" can be written "m/s", "m s−1", "m·s−1" or
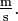 Only one solidus should be used; i.e., "kg·m−1·s−2" is preferable to "kg/m/s2", and "kg/m·s2" is something else. Many computer users will type the / character provided on computer keyboards, which in turn produces the Unicode character
Only one solidus should be used; i.e., "kg·m−1·s−2" is preferable to "kg/m/s2", and "kg/m·s2" is something else. Many computer users will type the / character provided on computer keyboards, which in turn produces the Unicode character U+002F, which is named solidus but is distinct from the Unicode solidus character,U+2044. - In Chinese, Japanese, and Korean language computing (CJK), some of the commonly-used units, prefix-unit combinations, or unit-exponent combinations have been allocated predefined single characters taking up a full square. Unicode includes these in its CJK Compatibility and Letterlike Symbols subranges for back compatibility, without necessarily recommending future usage.
- When writing dimensionless quantities, the terms 'ppb' (parts per billion) and 'ppt' (parts per trillion) are recognised as language-dependent terms, since the value of billion and trillion can vary from language to language. SI, therefore, recommends avoiding these terms.[16] However, no alternative is suggested by the International Bureau of Weights and Measures (BIPM).
Spelling variations
- The official US spellings for deca, metre, and litre are deka, meter, and liter, respectively.[17]
- In some English-speaking countries, the unit ampere is often shortened to amp (singular) or amps (plural) in informal writing.
Conversion factors
The relationship between the units used in different systems is determined by convention or from the basic definition of the units. Conversion of units from one system to another is accomplished by use of a conversion factor. There are several compilations of conversion factors; see, for example, Appendix B of NIST SP 811.[11]
Length, mass and temperature convergence
Specific gravity is commonly expressed in SI units or in reference to water. Since a cube with sides of 1 dm has volume of 1 dm3, which is 1 L and, when filled with water, has a mass of 1 kg, water has an approximate specific gravity of 1 kg/L, which is equal to 1 g/cm3 and 1 t/m3, and will freeze at 0 °C at 1 atmosphere of pressure.
Note that this is only an approximate definition of the kg, as the volume of water can change with temperature; the actual definition is based on a specific platinum-iridium cylinder held in a vault at the BIPM in Sèvres, France.
Cultural issues
The near-worldwide adoption of the metric system as a tool of economy and everyday commerce was based to some extent on the lack of customary systems in many countries to adequately describe some concepts, or as a result of an attempt to standardise the many regional variations in the customary system. International factors also affected the adoption of the metric system, as many countries increased their trade. For use in science, it simplifies dealing with very large and small quantities, since it lines up so well with the decimal numeral system.
Many units in everyday and scientific use are not derived from the seven SI base units (metre, kilogram, second, ampere, kelvin, mole, and candela) combined with the SI prefixes. In some cases these deviations have been approved by the BIPM.[18] Some examples include:
- The many units of time — minute (min), hour (h), day (d) — in use besides the SI second, and are specifically accepted for use according to table 6.[19]
- The year is specifically not included but has a recommended conversion factor.[20]
- The Celsius temperature scale; kelvins are rarely employed in everyday use.
- Electric energy is often billed in kilowatt-hours instead of megajoules.
- The nautical mile and knot (nautical mile per hour) used to measure travel distance and speed of ships and aircraft (1 International nautical mile = 1852 m or approximately 1 minute of latitude). In addition to these, Annex 5 of the Convention on International Civil Aviation permits the "temporary use" of the foot for altitude.
- Astronomical distances measured in astronomical units, parsecs, and light-years instead of, say, petametres (a light-year is about 9.461 Pm or about 9 461 000 000 000 000 m).
- Atomic scale units used in physics and chemistry, such as the ångström, electron volt, atomic mass unit and barn.
- Some physicists prefer the centimetre-gram-second (CGS) units, with their associated non-SI electric units.
- In some countries the informal cup measurement has become 250 ml. Likewise, a 500 g "metric pound" is used in many countries. Liquids, especially alcoholic ones, are often sold in units whose origins are historical (for example, pints for beer and cider in glasses in the UK — although pint means 568 ml; champagne in Jeroboams in France).
- A metric mile of 10 km is used in Norway and Sweden. The term metric mile is also used in some English speaking countries for the 1500 m foot race.
- In the US blood glucose measurements are recorded in milligrams per decilitre (mg/dL); in Canada, Australia, New Zealand, Oceania and Europe, the standard is millimole per litre (mmol/L) or mM (millimolar).
- Blood pressure is measured in mmHg instead of Pa.
The fine-tuning that has happened to the metric base-unit definitions over the past 200 years, as experts have tried periodically to find more precise and reproducible methods, does not affect the everyday use of metric units. Since most non-SI units in common use, such as the US customary units, are nowadays defined in SI units,[21] any change in the definition of the SI units results in a change of the definition of the older units, as well.
Trade
The European Union has a directive[22] banning non-SI markings after 31 December 2009 on any goods imported into the European Union. This applies to all markings on products, enclosed directions and papers, packaging and advertisements. On September 11, 2007, the EU announced that the United Kingdom would be exempted from this directive and imperial measurements would still be permitted indefinitely alongside with the metric system as supplementary indications.[23]
See also
|
|
|
Organisations |
||
|
|
|
Standards and conventions |
||
|
|
|
|||||||||||||||||
References
- ↑ Bureau International des Poids et Mesures
- ↑ Official BIPM definitions
- ↑ An extensive presentation of the SI units is maintained on line by NIST, including a diagram of the interrelations between the derived units based upon the SI units. Definitions of the basic units can be found on this site, as well as the CODATA report listing values for special constants such as the electric constant, the magnetic constant and the speed of light, all of which have defined values as a result of the definition of the metre and ampere.
– CODATA reportIn the International System of Units (SI) (BIPM, 2006), the definition of the meter fixes the speed of light in vacuum c0, the definition of the ampere fixes the magnetic constant (also called the permeability of vacuum) μ0, and the definition of the mole fixes the molar mass of the carbon 12 atom M(12C) to have the exact values given in the table [Table 1, p.7]. Since the electric constant (also called the permittivity of vacuum) is related to μ0 by ε0 = 1/μ0c02, it too is known exactly.
- ↑ SI Practical Realization brochure
- ↑ Ambler Thompson and Barry N. Taylor, (2008), Guide for the Use of the International System of Units (SI), (Special publication 811), Gaithersburg, MD: National Institute of Standards and Technology, p. 3, footnote 2.
- ↑ Bureau International des Poids et Mesures (2006). The International System of Units (SI) (8th Edn). ISBN 92-822-2213-6. p. 111.
- ↑ "The name "kilogram"". Retrieved on 2006-07-25.
- ↑ SI Brochure
- ↑ Barry N. Taylor & Ambler Thompson Ed.. The International System of Units (SI). Gaithersburg, MD: National Institute of Standards and Technology. pp. 23. http://physics.nist.gov/Pubs/SP330/sp330.pdf. Retrieved on 2008-06-18.
- ↑ 10.0 10.1 Bureau International des Poids et Mesures (2006). "The International System of Units (SI)". 8th ed.. Retrieved on 2008-02-13. Chapter 5.
- ↑ 11.0 11.1 Ambler Thompson & Barry N. Taylor (2008). "NIST Special Publication 811: Guide for the Use of the International System of Units (SI)". National Institute of Standards and Technology. Retrieved on 2008-06-18.
- ↑ James M. Turner (2008-5-9). "Interpretation of the International System of Units (the Metric System of Measurement) for the United States". Government Printing Office. Retrieved on 2008-06-18.
- ↑ Taylor, B. N.. "NIST Guide to SI Units - Rules and Style Conventions". National Institute of Standards and Technology. Retrieved on 2007-04-12.
- ↑ "The International System of Units (SI)". International Bureau of Weights and Measures (BIPM). Retrieved on 2008-04-18.
- ↑ Barry N. Taylor, Ed.. The International System of Units (SI). Washington, DC: National Institute of Standards and Technology. pp. 30. http://physics.nist.gov/Pubs/SP330/sp330.pdf. Retrieved on 2007-10-15.
- ↑ http://www.bipm.org/en/si/si_brochure/chapter5/5-3-7.html
- ↑ "The International System of Units" iii. Retrieved on 2008-05-27.
- ↑ BIPM - Table 8
- ↑ BIPM - Table 6
- ↑ NIST Guide to SI Units - Appendix B9. Conversion Factors
- ↑ Mendenhall, T. C. (1893). "Fundamental Standards of Length and Mass". Reprinted in Barbrow, Louis E. and Judson, Lewis V. (1976). Weights and measures standards of the United States: A brief history (NBS Special Publication 447). Washington D.C.: Superintendent of Documents. Viewed 23 August 2006 at http://physics.nist.gov/Pubs/SP447/ pp. 28–29.
- ↑ Council Directive 80/181/EEC of 20 December 1979 on the approximation of the laws of the Member States relating to units of measurement and on the repeal of Directive 71/354/EEC, as amended with Directive 89/617/EEC (which changed the cutoff date in article 3.2 to 31 December 1999) and Directive 1999/103/EC (which further changed the date to 31 December 2009). Retrieved on 2006-07-24.
- ↑ BBC NEWS | UK | EU gives up on 'metric Britain'
Further reading
- International Union of Pure and Applied Chemistry (1993). Quantities, Units and Symbols in Physical Chemistry, 2nd edition, Oxford: Blackwell Science. ISBN 0-632-03583-8. Electronic version.
- Unit Systems in Electromagnetism
External links
- Official
- BIPM Bureau International des Poids et Mesures (SI maintenance agency) (home page)
- BIPM brochure (SI reference)
- ISO 1000:1992 SI units and recommendations for the use of their multiples and of certain other units
- NIST Official Publications
- Weights and Measures Act, Canada
- IEEE/ASTM SI 10-2002 Standard for Use of the International System of Units (SI): The Modern Metric System (ANSI approved, joint IEEE/ASTM standard)
- Rules for SAE Use of SI (Metric) Units
- National Physical Laboratory, UK
- Information
- International System of Units at the Open Directory Project
- EngNet Metric Conversion Chart Online Categorised Metric Conversion Calculator
- A Practical Guide to the International System of Units
- History
- LaTeX SIunits package manual gives a historical background to the SI system.
- Research
- Pro-metric pressure groups
- Pro-customary measures pressure groups
- Pro-customary measures groups at the Open Directory Project