Propionic acid
| Propionic acid | |
|---|---|
 |
 |
 |
|
| IUPAC name | propanoic acid |
| Other names | ethanecarboxylic acid |
| Identifiers | |
| CAS number | 79-09-4 |
| RTECS number | UE5950000 |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
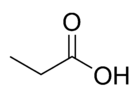
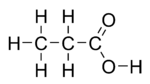
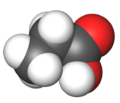
| Molecular formula | CH3CH2COOH |
| Molar mass | 74.08 g/mol |
| Appearance | colourless liquid |
| Density | 0.99 g/cm³, liquid |
| Melting point |
-21 °C (252 K) |
| Boiling point |
141 °C (414 K) |
| Solubility in water | miscible |
| Acidity (pKa) | 4.88 |
| Viscosity | 10 mPa·s |
| Structure | |
| Dipole moment | 0.63 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Corrosive |
| NFPA 704 |
 2
3
0
|
| R-phrases | R34 |
| S-phrases | (S1/2), S23, S36, S45 |
| Flash point | 54°C |
| Related compounds | |
| Other anions | sodium propionate |
| Related carboxylic acids | acetic acid butyric acid |
| Related compounds | 1-propanol propionaldehyde methyl propionate propionic anhydride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Propionic acid (systematically named propanoic acid) is a naturally-occurring carboxylic acid with chemical formula CH3CH2COOH. In the pure state, it is a colorless, corrosive liquid with a pungent odor. The anion CH3CH2COO− as well as the salts and esters of propionic acid are known as propionates (or propanoates).
Contents |
History
Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid in various other ways, none of them realizing they were producing the same substance. In 1847, the French chemist Jean-Baptiste Dumas established that all the acids were the same compound, which he called propionic acid, from the Greek words protos = "first" and pion = "fat," because it was the smallest H(CH2)nCOOH acid that exhibited the properties of the other fatty acids, such as producing an oily layer when salted out of water and having a soapy potassium salt.
Properties
Propionic acid has physical properties intermediate between those of the smaller carboxylic acids, formic and acetic acid, and the larger fatty acids. It is miscible with water, but it can be removed from water by adding salt. As with acetic and formic acids, its vapor grossly violates the ideal gas law because it does not consist of individual propionic acid molecules, but instead of hydrogen bonded pairs of molecules. It also undergoes this pairing in the liquid state.
Propionic acid displays the general properties of carboxylic acids, and, like most other carboxylic acids, it can form amide, ester, anhydride, and chloride derivatives. It can undergo alpha-halogenation with bromine in the presence of PBr3 as catalyst (the HVZ reaction) to form CH3CHBrCOOH.
Production
In industry, propionic acid is main produced by the hydrocarboxylation of ethylene using nickel carbonyl as the catalyst:[1]
- RCH=CH2 + H2O + CO → CH3CH2CO2H
It is also produced by the aerobic oxidation of propionaldehyde. In the presence of cobalt or manganese ions, this reaction proceeds rapidly at temperatures as mild as 40-50°C:
- CH3CH2CHO + ½ O2 → CH3CH2COOH
Large amounts of propionic acid were once produced as a byproduct of acetic acid manufacture. Current world's largest producer is BASF, with approximately 80 ktpa production capacity.
Propionic acid is produced biologically as its coenzyme A ester, propionyl-CoA, from the metabolic breakdown of fatty acids containing odd numbers of carbon atoms, and also it the breakdown of some amino acids. Bacteria of the genus Propionibacterium produce propionic acid as the end product of their anaerobic metabolism. This class of bacteria is commonly found in the stomachs of ruminants and the sweat glands of humans, and their activity is partially responsible for the odor of both Swiss cheese and sweat.
Uses
Propionic acid inhibits the growth of mold and some bacteria. As a result, most propionic acid produced is used as a preservative for both animal feed and food for human consumption, and can be used as a preservative for Ballistics Gel. For animal feed, it is used either directly or as its ammonium salt. In human foods, especially bread and other baked goods, it is used as its sodium or calcium salt. Similar usage occurs in some of the older anti-fungal foot powders.
Propionic acid is also useful as a chemical intermediate. It can be used to modify synthetic cellulose fibers. It is also used to make pesticides and pharmaceuticals. The esters of propionic acid are sometimes used as solvents or artificial flavorings.
Safety
The chief danger from propionic acid is chemical burns that can result from contact with the concentrated liquid. In studies on laboratory animals, the only adverse health effect associated with long-term exposure to small amounts of propionic acid has been ulceration of the esophagus and stomach from consuming a corrosive substance. No toxic, mutagenic, carcinogenic, or reproductive effects have ever been observed. In the body, propionic acid is readily metabolized, so it does not bioaccumulate.
A recent publication by MacFabe and colleagues found that intraventricular infusions of propionic acid produced reversible behavior that was very similar to that seen in autism. Behaviors included: hyperactivity, dystonia, turning, retropulsion. In addition, the treated rats demonstrated caudate spiking and the progressive development of limbic kindled seizures. The abstract concludes that this may be an excellent animal model of certain types of autism.[2]
Metabolism
The metabolism of propionic acid begins with its conversion to propionyl coenzyme A (propionyl-CoA), the usual first step in the metabolism of carboxylic acids.
Since propionic acid has three carbons, propionyl-CoA can enter neither beta oxidation nor the citric acid cycle
In most vertebrates, propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerised to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B12-dependent enzyme. Succinyl-CoA is an intermediate of the citric acid cycle and can be readily incorporated there.
In propionic acidemia, propionate acts as a metabolic toxin in liver cells by accumulating in mitochondria as propionyl-CoA and its derivative, methylcitrate, two tricarboxylic acid cycle inhibitors. Propionate is metabolized oxidatively by glia, which suggests astrocytic vulnerability in propionic acidemia when intramitochondrial propionyl-CoA may accumulate. Propionic acidemia may alter both neuronal and glial gene expression by affecting histone acetylation.[2][3]
Human occurrence
The human skin is host to a species of bacteria known as Propionibacterium acnes, which is named after its ability to produce propionic acid. This bacteria lives mainly in the sebaceous glands of the skin and is one of the principal causes of acne.
References
- ↑ W. Bertleff, M. Roeper, X. Sava, “Carbonylation” in Ullmann’s Encyclopedia of Chemical Technology Wiley-VCH: Weinheim, 2003. DOI: 10.1002/14356007.a05 217.
- ↑ 2.0 2.1 D. F. MacFabe, D. P. Cain, K. Rodriguez-Capote, A. E. Franklin, J. E. Hoffman, F. Boon, A. R. Taylor, M. Kavaliers and K.-P. Ossenkopp (2007). "Neurobiological effects of intraventricular propionic acid in rats: Possible role of short-chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders". Behavioral Brain Research 176 (1): 149–169. doi:.
- ↑ N. H. T. Nguyen, C. Morland, S. Villa Gonzalez, F. Rise, J. Storm-Mathisen, V. Gundersen, B. Hassel (2007). "Propionate increases neuronal histone acetylation, but is metabolized oxidatively by gli. Relevance for propionic acidemia". Journal of Neurochemistry 101 (3): 806–814. doi:.