Polyester

Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate (PET). Polyesters include naturally-occurring chemicals, such as in the cutin of plant cuticles, as well as synthetics such as polycarbonate and polybutyrate.
Polyesters may be produced in numerous forms such as sheets and three-dimensional shapes. Polyesters as thermoplastics may change shape after the application of heat. While combustible at high temperatures, polyesters tend to shrink away from flames and self-extinguish upon ignition. Polyester fibers have high tenacity and E-modulus as well as low water absorption and minimal shrinkage in comparison with other industrial fibers.
Woven polyester fabrics are used in consumer apparel and home furnishings such as bed sheets, bedspreads, curtains and draperies. Similarly, industrial polyesters are used in tyre reinforcements, ropes, fabrics for conveyor belts, safety belts, coated fabrics and plastic reinforcements with high energy absorption. Polyester fiberfills are also used to stuff pillows, comforters and cushion padding.
Polyester fabrics are claimed to have a "less natural" feel when compared to similarly-woven fabrics made from natural fibers (i.e. cotton in textile uses). However, polyester fabrics may exhibit other advantages over natural fabrics, such as improved wrinkle resistance. As a result, polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties.

Polyesters are also used to make bottles, films, tarpaulin, canoes, liquid crystal displays, holograms, filters, dielectric film for capacitors, film insulation for wire and insulating tapes.
Liquid crystalline polyesters are among the first industrially-used liquid crystalline polymers. They are used for their mechanical properties and heat-resistance. These traits also important in their application as an abradable seal in jet engines.
Thermosetting polyesters are used as casting materials, and chemosetting polyester resins are used as fiberglass laminating resins and non-metallic auto-body fillers. Fiberglass-reinforced unsaturated polyesters find wide application in bodies of yachts and as body parts of cars.
Polyesters are also widely used as a finish on high-quality wood products such as guitars, pianos and vehicle / yacht interiors. Burns Guitars, Rolls Royce and Sunseeker are a few companies that use polyesters to finish their products. Thixotropic properties of spray-applicable polyesters make them ideal for use on open-grain timbers, as they can quickly fill wood grain, with a high-build film thickness per coat. Cured polyesters can be sanded and polished to a high-gloss, durable finish.
Contents |
Polyester fiber properties
Mechanical properties
Energy absorption of chemical fiber reinforced plastics (impact, bending and tensile tests) Investigation of the practical requirements for measuring the energy absorption of composite materials, and development of a suitable method for carrying out such measurements. A number of dynamic testing methods for measuring the energy absorption of laminates are reviewed, including an impact bending test, repeated-impact tests, an impact tensile test, and a ram bending test. Also discussed are impact tests on plate laminates. Particular emphasis is placed in these studies on composites with a chemical fiber reinforcement. It is established that a relation exists between the quasi-static energy absorption of the fibers and the dynamic energy absorption of the composite. Composites with commercial polyester and polyamide fibers lead to the highest energy absorptions, in which case the testing apparatus has a significant effect.
Chemical properties
There are no known chemical properties of polyester.
The polyester industry
Basics
Polyester is a synthetic polymer made of purified terephthalic acid (PTA) or its dimethyl ester dimethyl terephthalate (DMT) and monoethylene glycol (MEG). With 18% market share of all plastic materials produced, it ranges third after polyethylene (33.5%) and polypropylene (19,5%).
The main raw materials are described as follows:
- Purified Terephthalic Acid – PTA – CAS-No.: 100-21-0
- Synonym: 1,4 Dibenzenedicarboxylic acid,
- Sum formula; C6H4(COOH)2 , mol weight: 166,13
- Dimethylterephthalate – DMT- CAS-No: 120-61-6
- Synonym: 1,4 Dibenzenedicarboxylic acid dimethyl ester
- Sum formula C6H4(COOCH3)2 , mol weight: 194,19
- Mono Ethylene Glycol – MEG – CAS No.: 107-21-1
- Synonym: 1,2 Ethanediol
- Sum formula: C2H6O2 , mol weight: 62,07
More information about polyester raw materials can be found for PTA [1],DMT [2] and MEG [3], at the webpage INCHEM "Chemical Safety Information from Intergovernmental Organizations".
To make a polymer of high molecular weight a catalyst is needed. The most common catalyst is antimony trioxide (or antimony tri acetate):
Antimony trioxide – ATO – CAS-No.: 1309-64-4 Synonym: non, mol weight: 291,51 Sum formula: Sb2O3
In 2008 about 10 000 t Sb2O3 were used to produce around 49 Mio t polyethylene terephthalate.
Polyester is described as follows:
Polyethylene Terephthalate CAS-No.: 25038-59-9 Synonym / abbreviations: polyester, PET, PES Sum Formula: H-[C10H8O4]-n=60-120 OH, mol unit weight: 192,17
There are several reasons for the importance of PTA:
- The relatively easy accessible raw materials PTA or DMT and MEG
- The very well understood and described simple chemical process of polyester synthesis
- The low toxicity level of all raw materials and side products during production and processing
- The possibility to produce PET in a closed loop at low emissions to the environment
- The outstanding mechanical and chemical properties of polyester
- The recycle ability
- The wide variety of intermediate and final products made of polyester
In table 1 the estimated world polyester production for textile polyester, bottle polyester resin, film polyester mainly for packaging and specialty polyesters for engineering plastics, which are the main fields of application, can be seen. According to this table, the world's total polyester production might exceed 50 million tons per annum before the year 2010.
Table 1: World polyester production
| Market size per year | ||
|---|---|---|
| Product Type | 2002 [Mio t/a] | 2008 [Mio t/a] |
| Textile-PET | 20 | 39 |
| Resin, Bottle/A-PET | 9 | 16 |
| Film-PET | 1.2 | 1.5 |
| Special Polyester | 1 | 2.5 |
| TOTAL | 31.2 | 49 |
Raw material producer
The raw materials PTA, DMT and MEG are mainly produced by large chemical companies which are sometimes integrated down to the crude oil refinery where p-xylene is the base material to produce PTA and liquefied petroleum gas (LPG) is the base material to produce MEG.
Large PTA producers are for instance BP, Reliance, Sinopec, SK-Chemicals, Mitsui and Eastman Chemicals. MEG production is in the hand of about 10 global players which are headed by MEGlobal a JV of DOW and PIC Kuweit followed by Sabic.
Among the world's largest polyester producers are the following companies:
Artenius, Advansa, DAK, DuPont, Eastman/Voridian, Hyosung, Huvis, Indorama, Invista, Jiangsu Hengli Chemical Fiber, Jiangsu Sanfangxian Industry, M&G Group, Mitsui, Mitsubishi, NanYa Plastics, Reichhold, Reliance, Rongsheng, Sabic, Teijin, Toray, Trevira, Tuntex, Wellman, Yizheng Sinopec, Zhejiang Hengi Polymerization.
With more than 500 plants in China, about half of the world production originates in that country. More information about polyester in China can be found under the web site of China Chemical Fiber Economic Information Network [4].
Polyester processing
After the first stage of polymer production in the melt phase, the product stream divides into two different application areas which are mainly textile applications and packaging applications. In figure 2 the main applications of textile and packaging polyester are listed.
Table 2: Textile and packaging polyester application list
| POLYESTER-BASED POLYMER (MELT or PELLET) | |
|---|---|
| Textile | Packaging |
| Staple fiber (PSF) | Bottles for CSD, Water, Beer, Juice, Detergents |
| Filaments POY, DTY, FDY | A-PET Film |
| Technical yarn and tire cord | Thermoforming |
| Non-woven and spunbond | BO-PET Biaxial oriented Film |
| Mono-filament | Strapping |
Abbreviations: PSF = Polyester Staple Fiber; POY = Partially Oriented Yarn; DTY = Draw Textured Yarn; FDY = Fully Drawn Yarn; CSD = Carbonated Soft Drink; A-PET = Amorphous Polyester Film; BO-PET = Biaxial Oriented Polyester Film;
A comparable small market segment (<< 1 Million t/a) of polyester is used to produce engineering plastics and masterbatch.
In order to produce the polyester melt with a high efficiency, high-output processing steps like staple fiber (50–300 t/d per spinning line) or POY /FDY (up to 600 t/d split into about 10 spinning machines) are meanwhile more and more horizontal, integrated, direct processes. This means the polymer melt is directly converted into the textile fibers or filaments without the common step of pelletizing. We are talking about full horizontal integration when polyester is produced at one site starting from crude oil or distillation products in the chain oil -> benzene -> PX -> PTA -> PET melt -> fiber / filament or bottle-grade resin. Such integrated processes are meanwhile established in more or less interrupted processes at one production site. Eastman Chemicals introduced at first the idea to close the chain from PX to PET resin with their so-called INTEGREX® process. The capacity of such horizontal, integrated productions sites is >1000 t/d and can easily reach 2500 t/d.
Besides the above mentioned large processing units to produce staple fiber or yarns, there are ten thousands of small and very small processing plants, so that one can estimate that polyester is processed and recycled in more than 10 000 plants around the globe. This is without counting all the companies involved in the supply industry, beginning with engineering and processing machines and ending with special additives, stabilizers and colors. This is a gigantic industry complex and it is still growing by 4–8% per annum, depending on the world region. Useful information about the polyester industry can be found under [5] where a “Who is Producing What in the Polyester Industry” is gradually being developed.
Synthesis
Synthesis of polyesters is generally achieved by a polycondensation reaction. See "condensation reactions in polymer chemistry". The General equation for the reaction of a diol with a diacid is : (n+1) R(OH)2 + n R´(COOH)2 ---> HO[ROOCR´COO]nROH + 2n H2O
Azeotrope esterification
In this classical method, an alcohol and a carboxylic acid react to form a carboxylic ester. To assemble a polymer, the water formed by the reaction must be continually removed by azeotrope distillation.
Alcoholic transesterification
See main article on transesterification.
O
\\
C - OCH3 + OH[Oligomer2]
/
[Oligomer1]
|
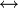 |
O
\\
C - O[Oligomer2] + CH3OH
/
[Oligomer1]
|
| (ester-terminated oligomer + alcohol-terminated oligomer) | (larger oligomer + methanol) |
Acylation (HCl method)
The acid begins as an acid chloride, and thus the polycondensation proceeds with emission of hydrochloric acid (HCl) instead of water. This method can be carried out in solution or as an enamel.
- Silyl method
- In this variant of the HCl method, the carboxylic acid chloride is converted with the trimethyl silyl ether of the alcohol component and production of trimethyl silyl chloride is obtained
Acetate method (esterification)
- Silyl acetate method
Ring-opening polymerization
Aliphatic polyesters can be assembled from lactones under very mild conditions, catalyzed anionically, cationically or metallorganically.
Thermosetting Thermosetting resins are generally copolymers of unsaturated polyesters with styrene. Polyester saturation is governed through the use of maleic acid or fumaric acid. In vinyl esters, saturation (or lack thereof) is found in the alcohol group of the polyester. The double bond of unsaturated polyester reacts with styrene resulting in a 3-D cross-linked structure. This structure acts as a thermoset. The cross-linking is initiated through an exothermic reaction involving an organic peroxide, such as methyl ethyl ketone peroxide or benzoyl peroxide.
References
- Textiles, by Sara Kadolph and Anna Langford. 8th Edition, 1998.
|
|||||
|
||||||||||||||||||
