Acrylic glass
| Acrylic glass | |
|---|---|
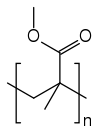 |
|
| Other names | poly(methyl methacrylate) (PMMA) methyl methacrylate resin |
| Identifiers | |
| CAS number | 9011-14-7 |
| SMILES |
|
| Properties | |
| Molecular formula | (C5O2H8)n |
| Molar mass | varies |
| Density | 1.19 g/cm³ |
| Melting point |
130–140 °C (265–285 °F) |
| Boiling point |
200.0 °C (392 °F) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Poly(methyl methacrylate) (PMMA) poly(methyl 2-methylpropenoate) is a thermoplastic and transparent plastic. Chemically, it is the synthetic polymer of methyl methacrylate. It is sold by the tradenames Plexiglas, Vitroflex, Limacryl, R-Cast, Per-Clax, Perspex, Plazcryl, Acrylex, Acrylite, Acrylplast, Altuglas, Polycast, Oroglass and Lucite and is commonly called acrylic glass, simply acrylic or plexiglas. Acrylic, or acrylic fiber, can also refer to polymers or copolymers containing polyacrylonitrile. The material was developed in 1928 in various laboratories and was brought to market in 1933 by Rohm and Haas Company.
PMMA is often used as an alternative to glass, and in competition with polycarbonate (PC). It is often preferred because of its moderate properties, easy handling and processing, and low cost, but behaves in a brittle manner when loaded, especially under an impact force. To produce 1 kg of PMMA, about 2 kg of petroleum is needed. PMMA ignites at 460°C and burns completely to form only carbon dioxide and water.
Contents |
History
The first acrylic acid was created in 1843. Methacrylic acid, derived from acrylic acid, was formulated in 1865. The reaction between methacrylic acid and methyl alcohol results in the ester methyl methacrylate. The German chemists Fittig and Paul discovered in 1877 the polymerization process that turns methyl methacrylate into polymethyl methacrylate. In 1933 the German chemist Otto Röhm patented and registered the brand name PLEXIGLAS. In 1936 the first commercially viable production of acrylic safety glass began. During World War II acrylic glass was used for submarine periscopes, and windshields, canopies, and gun turrets for airplanes. [1]
Synthesis
PMMA is routinely produced by emulsion polymerization, solution polymerization and bulk polymerization. Generally radical initiation is used (including living polymerization methods), but anionic polymerization of PMMA can also be performed.
Processing
Thermoplastic PMMA is typically processed at 240–250 °C. All common molding processes may be used, including injection molding, compression molding and extrusion. The highest quality PMMA sheets are produced by cell casting, but in this case, the polymerization and molding steps occur concurrently. The strength of the material is higher than molding grades owing to its extremely high molecular mass. Rubber toughening has been used to increase the strength of PMMA owing to its brittle behavior in response to applied loads.
PMMA can be joined using cyanoacrylate cement (so-called "Superglue"), with heat (melting), or by using solvents such as di- or trichloromethane to dissolve the plastic at the joint which then fuses and sets, forming an almost invisible weld.
Scratches may easily be removed by polishing or by heating the surface of the material.
Laser cutting may be used to form intricate designs from PMMA sheets. PMMA vaporises to gaseous compounds (including its monomers) upon laser cutting, so a very clean cut is made, and cutting is performed very easily. In this respect PMMA has an advantage over competing polymers such as polystyrene and polycarbonate, which require higher laser powers and give more messy and charred laser cuts.
Properties
PMMA:
- has a density of 1,150–1,190 kg/m3. This is less than half the density of glass, and similar to that of other plastics.
- has a good impact strength higher than that of glass or polystyrene, but significantly lower than that of polycarbonate or engineering polymers. In the majority of applications, it will not shatter but instead breaks into large dull pieces.
- is softer and more easily scratched than glass. Scratch-resistant coatings (which may also have other functions) are often added to PMMA sheets.
- transmits up to 98% of visible light (3 mm thickness), and gives a ~4% reflection from each of its surfaces on account of its refractive index of 1.4893 to 1.4899.


- filters ultraviolet (UV) light at wavelengths below ~300 nm. Some manufacturers[2] add coatings or additives to PMMA to improve absorption in the 300–400 nm range.
- allows infrared light of up to 2800 nm wavelength to pass. IR of longer wavelengths, up to 25,000 nm, are essentially blocked. Special formulations of colored PMMA exist to allow specific IR wavelengths to pass while blocking visible light (for remote control or heat sensor applications, for example).
- has excellent environmental stability compared to other plastics such as polycarbonate, and is therefore often the material of choice for outdoors applications.
- has poor resistance to solvents, as it swells and dissolves easily. It also has poor resistance to many other chemicals on account of its easily hydrolyzed ester groups.
Modification of properties
Pure poly(methyl methacrylate) homopolymer is rarely sold as an end product, since it is not optimized for most applications. Rather, modified formulations with varying amounts of other comonomers, additives, and fillers are created for uses where specific properties are required. For example,
- A small amount of acrylate comonomers are routinely used in PMMA grades destined for heat-processing, since this stabilizes the polymer to depolymerization ("unzipping") during processing.
- Comonomers such as butyl acrylate are often added to improve impact strength.
- Comonomers such as methacrylic acid can be added to increase the glass transition temperature of the polymer for higher temperature use such as in lighting applications.
- Plasticizers may be added to improve processing properties, lower the glass transition temperature, or improve impact properties.
- Dyes may be added to give color for decorative applications, or to protect against (or filter) UV light.
- Fillers may be added to improve cost-effectivness.
Related polymer poly(methyl acrylate)
The polymer of methyl acrylate, PMA or poly(methyl acrylate), is similar to poly(methyl methacrylate), except for the lack of methyl groups on the backbone carbon chain.[3] PMA is a soft white rubbery material that is softer than PMMA because its long polymer chains are thinner and smoother and can more easily slide past each other.
Uses
PMMA or Acrylic is a versatile material and has been used in a wide range of fields and applications.
Impact resistant substitute for glass
- PMMA Acrylic glass is commonly used for constructing residential and commercial aquariums.
- PMMA is used in the lenses of exterior lights of automobiles. [4]
- The spectator protection in ice hockey stadiums is made from PMMA.
- Motorcycle helmet visors
- Police vehicles for riot control often have the regular glass replaced with acrylic to protect the occupants from thrown objects.
- Acrylic is used for viewing ports and even complete hulls of submersibles, such as the Alicia submarine's viewing spheres and the Bathyscaphe Trieste's windows.
- Polycast acrylic sheet is the most widely used material in aircraft transparencies (windows). In applications where the aircraft is pressurized, stretched acrylic is used.
- Acrylic is an important material in the making of certain lighthouse lenses.[5]
Daylight redirection
- Laser cut acrylic panels have been used to redirect sunlight into a light pipe and, from there, to spread it into a room.[6] Their developers Veronica Garcia Hansen, Ken Yeang, and Ian Edmonds were awarded the Far East Economic Review Innovation Award in bronze for this technology in 2003.[7][8]
- Attenuation being quite strong for distances over one meter (more than 90% intensity loss for a 3000k source[9]), acrylic broadband light guides are then dedicated mostly to decorative uses.
- Pairs of acrylic sheets with a layer of microreplicated prisms between the sheets can have reflective and refractive properties that let them redirect part of incoming sunlight in dependence on its angle of incidence. Such panels act as miniature light shelves. Such panels have been commercialized for purposes of daylighting, to be used as a window or a canopy such that sunlight descending from the sky is directed to the ceiling or into the room rather than to the floor. This can lead to a higher illumination of the back part of a room, in particular when combined with a white ceiling, while having a slight impact on the view to the outside compared to normal glazing.[10][11]
Medical technologies and implants
- PMMA has a good degree of compatibility with human tissue, and can be used for replacement intraocular lenses in the eye when the original lens has been removed in the treatment of cataracts. Historically, hard contact lenses were frequently made of this material. Soft contact lenses are often made of a related polymer, where acrylate monomers containing one or more hydroxyl groups make them hydrophilic.
- In orthopaedics, PMMA bone cement is used to affix implants and to remodel lost bone. It is supplied as a powder with liquid methyl methacrylate (MMA). When mixed these yield a dough-like cement that gradually hardens. Surgeons can judge the curing of the PMMA bone cement by pressing their thumb on it. Although PMMA is biologically compatible, MMA is considered to be an irritant and a possible carcinogen. PMMA has also been linked to cardiopulmonary events in the operating room due to hypotension. [12] Bone cement acts like a grout and not so much like a glue in arthroplasty. Although sticky, it does not bond to either the bone or the implant, it primarily fills the spaces between the prosthesis and the bone preventing motion. A big disadvantage to this bone cement is that it heats to quite a high temperature while setting and because of this it kills the bone in the surrounding area. It has a Young's modulus between cancellous bone and cortical bone. Thus it is a load sharing entity in the body not causing bone resorption. [13]
- Dentures are often made of PMMA, and can be colour-matched to the patient's teeth & gum tissue. In cosmetic surgery, tiny PMMA microspheres suspended in some biological fluid are injected under the skin to reduce wrinkles or scars permanently.
Artistic and aesthetic uses
- Acrylic paint essentially consists of PMMA suspended in water; however since PMMA is hydrophobic, a substance with both hydrophobic and hydrophilic groups needs to be added to facilitate the suspension.
- Modern furniture makers, especially in the 1960s and 1970s, seeking to give their products a space age aesthetic incorporated Lucite and other PMMA products into their designs, especially office chairs. Many other products (for example, guitars) are sometimes made with acrylic glass to make the commonly opaque objects translucent.
- Perspex has been used as a surface to paint on, for example by Salvador Dalí.
- Diasec is a process which uses acrylic glass as a substitute for normal glass in picture framing. This is done for its relatively inexpensive cost, light weight, shatter-resistant nature, aesthetic reasons and for the fact that it can be ordered in larger sizes than standard picture-framing glass.
- From approximately the 1960s onward, sculptors and glass artists began using acrylics, especially taking advantage of the material's flexibility, light weight, cost and its capacity to refract and filter light.
- Sometimes used to make a Deal toy (in the world of finance or investment banking).
- Used in contact juggling.
Other uses


- PMMA is used as a shield to stop beta radiation emitted from radioisotopes.
- PMMA was used in laserdisc optical media. (CDs and DVDs use the more expensive polycarbonate for higher impact resistance)
- PMMA-based optical media is also under development for the "TeraDisc" next-generation 3D optical data storage solution by Mempile.[14]
- Artificial fingernails are made of acrylic.
- In the 1960s, luthier Dan Armstrong developed a line of electric guitars and basses whose bodies were made completely of acrylic. These instruments were marketed under the Ampeg brand. Ibanez [15] and BC Rich have also made acrylic guitars.
- Recently a blacklight-reactive tattoo ink using PMMA microcapsules was developed. This ink is reportedly safe for use, and claims to be Food and Drug Administration (FDA) approved for use on wildlife that may enter the food supply.
- In semiconductor research and industry, PMMA aids as a resist in the electron beam lithography process. A solution consisting of the polymer in a solvent is used to spin coat silicon and other semiconducting and semi-insulating wafers with a thin film. Patterns on this can be made by an electron beam (using an electron microscope), deep UV light (shorter wavelength than the standard photolithography process), or X-rays. Exposure to these creates chain scission or (de-cross-linking) within the PMMA, allowing for the selective removal of exposed areas by a chemical developer, making it a positive photoresist. PMMA's advantage is that it allows for extremely high resolution (nanoscale) patterns to be made. It is an invaluable tool in nanotechnology.
- Small strips of PMMA are used as dosimeter devices during the Gamma Irradiation process. The optical density of PMMA changes as the Gamma dose increases and can be measured with a spectrophotometer.
- It is used as a light guide for the backlights in TFT-LCDs.
- Plastic optical fiber used for short distance communication is made from PMMA, and perfluorinated PMMA, clad with fluorinated PMMA, in situations where its flexibility and cheaper installation costs outweigh its poor heat tolerance and higher attenuation over glass fiber.
- Sheets of PMMA are commonly used in the sign industry to make flat cut out letters in thicknesses typically varying from 0.125" to 1". These letters may be used alone to represent a company's name and/or logo, or they may be a component of channel letters which are neon or LED illuminated sign "can" commonly seen worldwide. Acrylic's attractiveness, durability and resistance to warping makes it an ideal interior and exterior sign material.
- Ludwig-Musser makes a line of acrylic drums called Vistalites. They are well known as being used by Led Zeppelin drummer John Bonham.
- Ear stretching jewelry is also commonly formed out of PMMA, due to its inert nature and shatter-proof qualities. PMMA jewelry is fade-proof, odorless, durable, and easy to polish, giving it that modern stylish sheen.
- Apple Computer's Power Mac G4 Cube was made out of PMMA
- WWE's Elimination Chamber match has 4 "Containment Pods," each with a metal outlining and PMMA walls.
- Acrylic is also used extensively throughout the sign industry as a component of wall signs where it may be a backplate, painted on the surface or the backside, a faceplate with additional raised lettering or even photographic images printed directly to it, or a spacer to separate sign components. One of the most popular sheets is a non-glare, translucent which is sold in 1/16 or 1/8 in thicknesses.
- Acrylic is often used in the manufacturing of bongs used for smoking tobacco, herbs, or cannabis.
See also
- Other transparent plastics: polystyrene, polycarbonate
References
- ↑ "Acrylic Plastic: How Products are Made". 080515 enotes.com
- ↑ Altuglas International Plexiglas UF-3 UF-4 and UF-5 sheets
- ↑ Polymethyl acrylate and polyethyl acrylate, Encyclopædia Britannica
- ↑ Kutz, Myer, Handbook of Materials Selection. John Wiley & Sons 2002. pg 341
- ↑ http://www.terrypepper.com/lights/closeups/illumination/index.htm Terry Pepper, Seeing the Light, Illumination.]
- ↑ Ken Yeang:Light Pipes: An Innovative Design Device for Bringing Natural Daylight and Illumination into Buildings with Deep Floor Plan, Nomination for the Far East Economic Review Asian Innovation Awards 2003
- ↑ Lighting up your workplace — Queensland student pipes light to your office cubicle, May 9, 2005
- ↑ Kenneth Yeang, World Cities Summit 2008, June 23—25, 2008, Singapore
- ↑ Modeling Attenuation versus Length in Practical Light Guides, April 2005
- ↑ How Serraglaze works
- ↑ Glaze of light, Building Design Online, June 8, 2007
- ↑ American Journal of Neuroradiology, 23:601–604, April 2002.
- ↑ Miller, Review of Orthopaedics, 4th Edition, p 129.
- ↑ Slashdot | Plexiglass-like DVD to Hold 1TB of Data
- ↑ http://www.ibanezregister.com/Gallery/js/gal-js2k.htm and http://ibanez.com/eg/guitar.aspx?m=JEM20TH
External links
- Cutting Plexiglass
- 'Vessels' - a sculptural book made from perspex – created by Adele Outteridge and held by the Australian Library of Art, State Library of Queensland
|
|||||