Lipid bilayer

A lipids bilayer or two layer lipid membrane (2LLM) is a membrane made up from lipid molecules (usually phospholipids). The lipid bilayer is a critical component of all biological membranes, including cell membranes, and so is absolutely essential for all known life on Earth. Its essential structure was discovered in 1925 by two Dutch physicians, E.Gorter and F.Grendel, while they were comparing the surface area of human erythrocytes with that of the isolated lipids in a Langmuir-Blodgett trough. They found that the area of lipids from a known number of erythrocytes, when spread out on the trough, was just twice the calculated surface area of the erythrocytes. They concluded, correctly, that the membrane is two lipid molecules thick and proposed it is made of a bilayer.
Contents |
Structure and function

The structure of a bilayer explains its function as a barrier. Lipids are as the same as the nutral cell membrane, amphiphilic molecules since they consist of polar head groups and non-polar fatty acid tails. The bilayer is composed of two layers of lipids arranged so that their hydrocarbon tails face one another to form an oily core held together by the hydrophobic effect, while their charged heads face the aqueous solutions on either side of the membrane. The hydrophilic interfacial regions are saturated with water, while the lipophilic core region contains essentially no water. Because of the oily core of the bilayer, it is only permeable to small hydrophobic solutes (such as chloroform or ethanol), but has a very low permeability to polar inorganic compounds and ionic molecules. For a cell, this means that even small molecules, such as sugars and salts, are contained inside it.
Support for the existence of a lipid bilayer in cell membranes came with the discovery by Alec Bangham in 1965 that phospholipids, when introduced into an aqueous environment, spontaneously form liposomes. These are small balloons of lipid bilayer which can entrap polar molecules inside them. The major force driving the formation of lipid bilayers is the hydrophobic interaction between the tails and their repulsion by water. Within the interior of the membrane the hydrocarbon tails are arranged, on average, perpendicular to the plane of the membrane. The properties of the bilayer are influenced by a variety of factors, including the lipid composition, temperature and membrane pressure. The most important features are the nature of the lipid head groups and the length and degree of saturation of the hydrocarbon chains. For example, introduction of a cis-double bond into the carbon chain produces a kink which is difficult to pack with straight neighbors. This in turn leads to a more fluid hydrocarbon milieu: the more kinks there are, the greater the disorder and the more fluid the oily core becomes.
The interfacial regions of model phospholipid bilayers have a thickness of 8 to 10 Å, although they can be wider in biological membranes that include lipid molecules whose head groups have complex carbohydrates, found in the gangliosides or lipopolysaccharides [1]. The thickness of the hydrocarbon core region is about 27 Å for the model bilayer formed by DOPC lipid whose acyl chains have eighteen carbon atoms and a single double bond (di(C18:1)PC) [2]. This is close to the hydrophobic thickness of typical biological membranes measured by small angle X-ray scattering (27-32 Å) [3]. The thickness of a lipid bilayer increases by 0.8 Å per each additional carbon atom of the lipid tail in the liquid crystalline state, or by 1.1 Å in the gel state (these numbers should be multiplied by 2 because the bilayer has two leaflets). [4] [5]. In addition, the presence of the flat cholesterol molecule in the membrane tends to straighten out the hydrocarbon chains, causing two main effects: the membrane becomes even less permeable to small molecules and the thickness of the hydrocarbon region is increased.
The boundary between the hydrocarbon core region and the water-saturated interface is very narrow (~3Å) and defined by the effective concentration of water [6] that changes exponentially from nearly zero to ~2M [7]. The hydrocarbon boundary plane passes through the carbonyl groups of phospholipids. The phosphate groups of phospholipids are completely hydrated and situated ~5 Å outside the hydrophobic membrane boundaries. [8]
Lipid bilayers have certain elastic properties. Free bilayers have zero surface tension and, like liquids, do not support shear stress. However, they have a non-zero Young's modulus and bulk modulus. They also can be described as having an internal lateral pressure or "the spontaneous radius of curvature", which defines the tendency of the lipid molecules to form curved rather than planar surfaces. [9]
Bilayers in cell membranes and lipid asymmetry
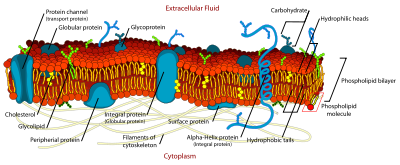
The membranes in cells are more complicated because they contain a variety of different classes of lipids, although the vast majority of the lipids are phospholipids and, in the case of higher cells, cholesterol. The main phospholipids are phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol and sphingomyelin. Glycolipids usually account for a few percent of the lipid molecules.
In mammalian cells, the phospholipids are not uniformly distributed between the two monolayers. As discovered in the human erythrocyte by M. Bretscher in 1972, the outer monolayer is largely composed of phosphatidyl-choline and sphingomyelin whereas the cytoplasmic monolayer contains the phosphatidyl-ethanolamine, phosphatidyl-serine and phosphatidyl-inositol. In addition, glycolipids are always found in the outer, non-cytoplasmic side. These asymmetries give the cell's bilayer properties different from those found in a liposome. For example, the negatively charged phospholipids, phosphatidyl-serine and phosphatidyl-inositol are found solely on the cytoplasmic side: this imparts a strong charge gradient across the bilayer which must affect the properties of transmembrane proteins and enzymes (note that membrane-spanning proteins are erroneously referred to as globular proteins in the opposite figure). The existence of lipid asymmetry in membranes depends on the very slow rate at which individual phospholipids can “flip-flop” — spontaneously move from one monolayer to the other — as first documented by R. Kornberg and H. McConnell in 1971. By contrast, the uncharged cholesterol readily flip-flops between monolayers and is approximately equally distributed on each side of the bilayer.
The asymmetry of phospholipids reflects their biosynthetic pathway. The four phosphatidyl-containing lipids are all synthesised on the cytoplasmic side of the endoplasmic reticulum, an internal membrane. The phosphatidylcholine, but not the other phospholipids, is then transported to the lumenal, non-cytoplasmic side, of that membrane by a flippase; this generates an asymmetry. Sphingomyelin is synthesised on, and incorporated into, the non-cytoplasmic side of the bilayer in the Golgi apparatus.
The asymmetry of the lipid bilayer has important biological functions. Thus, phosphatidylinositol and its phosphorylated derivatives are key signalling molecules inside the cell. Phosphatidylserine is used to indicate to scavenging macrophages that a dying cell should be removed: during apoptosis a phosphatidyl-serine specific flippase or scramblase is activated, revealing this lipid on the surface of the dying cell. This is recognised by macrophages which consume the corpse.
Bacteria also appear to have asymmetric bilayers, although the evidence is less clear-cut. The main lipids in many bacteria are phosphatidylethanolamine, phosphatidylglycerol and cardiolipin. In Bacillus megaterium, there appears to be about twice as much phosphatidylethanolamine in the cytoplasmic monolayer as in the outer one. This implies that much of the phosphatidylglycerol and cardiolipin may be in the outer monolayer.
Model lipid bilayers

Within a critical concentration range, certain lipids will self-organize in water to form a bilayer. Such membranes can be used in research, for example electrical experiments on the bilayer by using the patch clamp technique.
- Black BLM: a BLM over an aperture between two aqueous solutions. The advantage of this method is the ability to control the constituents of each side of the membrane. The disadvantage of this method is that it causes the membrane to be fairly unstable, and rupture is certain in a matter of hours.
- Supported BLM (s-BLM): a BLM covering a substrate surface (e.g. with patterned electrode). This method has the advantage of producing a stable membrane, which in some cases may last several days before rupturing. For example, preparation of supported lipid bilayer allowed to determine the formation of transmembrane pores (holes) around nanoparticles of approximately 1 to 20 nm in diameter.[10] The drawback of s-BLM method is that it is only possible to control the solution on the side of the membrane that is not in contact with the electrode, although studies show that a 1 nanometre-thick water layer forms between the membrane and the substrate, this is of insufficient volume for controlling the solution composition.
- Polymer-cushioned BLM: This technique is combination of the black-BLM and supported-BLM approaches. Electrodes are patterned over a surface and a polymer (typically a cellulose hydrogel) is coated on top of the electrodes. This polymer stabilises the membrane and acts as a spacer from the solid substrate complex.
Other lipid structures
See also Lipid polymorphism
Lipids can assume self-organized structures other than bilayers, depending on their concentration, chemical structure, and experimental conditions: micelles, monolayers, or different lipid mesophases.
References
- ↑ McIntosh T.J, Vidal A., and Simon S.A. 2002. The energetics of peptide-lipid interactions: modification by interfacial dipoles and cholesterol. In Current Topics in Membranes 52: 205-253.
- ↑ Lee, A.G. 2003 Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612: 1-40.
- ↑ Mitra, K., Ubarretxena-Belandia, Tim O'conner., Taguchi, T., Warren, G., and Engelman, D.M. 2004. Modulation of the bilayer thickness of exocytic pathway membranes by membrane proteins rather than cholesterol. Proc. Natl. Acad. Sci. 101: 4083–4088.
- ↑ Lewis, B.A. and Engelman, D.M. 1983a. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 166: 211–217.
- ↑ Dumas, F., Lebrun, M.C., and Tocanne, J.F. 1999. Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett. 458: 271–277.
- ↑ Marsh, D. 2001. Polarity and permeation profiles in lipid membranes. Proc. Natl. Acad. Sci. 98: 7777–7782.
- ↑ Marsh, D. 2002. Membrane water-penetration profiles from spin labels. Eur. Biophys. J. 31: 559–562.
- ↑ Nagle, J.F. and Tristram-Nagle, S. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta 1469: 159–195.
- ↑ McIntosh T.J. and Simon S.A. 2006. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 35: 177-198.
- ↑ 10.0 10.1 Y. Roiter, M. Ornatska, A. R. Rammohan, J. Balakrishnan, D. R. Heine, and S. Minko, Interaction of Nanoparticles with Lipid Membrane, Nano Letters, vol. 8, iss. 3, pp. 941-944 (2008).
External links
- Structure of Fluid Lipid Bilayers, from the Stephen White laboratory at University of California, Irvine
See also
- Biological membrane
- Vesicles
- Liposomes
- Elasticity of cell membranes
- Extracellular matrix
- Phospholipid
- Category: surfactants
- Protobiont
|
|||||