Pertussis
| Pertussis Classification and external resources |
|
 |
|
|---|---|
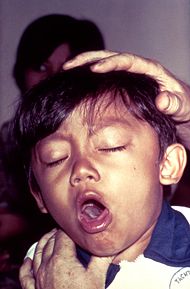
| A boy at a clinic with a cough. He was diagnosed with pertussis. | |
| ICD-10 | A37. |
| ICD-9 | 033 |
| DiseasesDB | 1523 |
| MedlinePlus | 001561 |
| eMedicine | emerg/394 ped/1778 |
| MeSH | D014917 |
Pertussis, also known as whooping cough, is a highly contagious disease caused by the bacterium Bordetella pertussis; it derived its name from the characteristic severe hacking cough followed by intake of breath that sounds like "whoop"; a similar, milder disease is caused by B. parapertussis.[1] Although many medical sources describe the whoop as "high-pitched", this is generally the case with infected babies and children only, not adults.[2]
Worldwide, there are 30–50 million pertussis cases and about 300,000 deaths per year. Despite generally high coverage with the DTP and DTaP vaccines, pertussis is one of the leading causes of vaccine-preventable deaths world-wide. Most deaths occur in young infants who are either unvaccinated or incompletely vaccinated; three doses of the vaccine are necessary for complete protection against pertussis. Ninety percent of all cases occur in the developing world. Children tend to catch it more than adults.
Contents |
Characterization
After a two day incubation period, pertussis in infants and young children is characterized initially by mild respiratory infection symptoms such as coughing, sneezing, and runny nose (catarrhal stage). After one to two weeks, the cough changes character, with an increase of coughing followed by an inspiratory "barking" sound (paroxysmal stage). Coughing fits may be followed by vomiting due to the sheer violence of the fit. In severe cases, the vomiting induced by coughing fits can lead to malnutrition and dehydration. The fits that do occur on their own can also be triggered by yawning, stretching, laughing or yelling. Triggering fits gradually diminish over one to two months during the convalescent stage. Other complications of the disease include pneumonia, encephalitis, pulmonary hypertension, and secondary bacterial superinfection.[3]
Transmission and diagnosis
Adults and adolescents are the primary reservoir for pertussis. Pertussis is spread by contact with airborne discharges from the mucous membranes of infected people, who are most contagious during the catarrhal stage. Because the symptoms during the catarrhal stage are nonspecific, pertussis is usually not diagnosed until the appearance of the characteristic cough of the paroxysmal stage. Methods used in laboratory diagnosis include culturing of nasopharyngeal swabs on Bordet-Gengou medium, polymerase chain reaction (PCR), immunofluorescence (DFA), and serological methods. The bacteria can be recovered from the patient only during the first three weeks of illness, rendering culturing and DFA useless after this period, although PCR may have some limited usefulness for an additional three weeks. For most adults and adolescents, who often do not seek medical care until several weeks into their illness, serology is often used to determine whether antibody against pertussis toxin or another component of B. pertussis is present at high levels in the blood of the patient.
Treatment
Treatment with an effective antibiotic (erythromycin or azithromycin) shortens the infectious period but does not generally alter the outcome of the disease; however, when treatment is initiated during the catarrhal stage, symptoms may be less severe. Three macrolides (erythromycin, azithromycin and clarithromycin) are used in the U.S. for treatment of pertussis; trimethoprim-sulfamethoxazole is generally used when a macrolide is ineffective or is contraindicated. Close contacts who receive appropriate antibiotics (chemoprophylaxis) during the 7–21 day incubation period may be protected from developing symptomatic disease. Close contacts are defined as anyone coming into contact with the respiratory secretions of an infected person in the 21 days before or after the infected person's cough began.
Isolation and identification
B. pertussis was isolated in pure culture in 1906 by Jules Bordet and Octave Gengou, who also developed the first serology and vaccine. The complete B. pertussis genome of 4,086,186 base pairs was sequenced in 2002.
Vaccines
History of pertussis vaccine development
Infection with pertussis induces immunity, but not lasting protective immunity, and a second attack is possible.[4] Efforts to develop an inactivated whole-cell pertussis vaccine began soon after B. pertussis was grown in pure culture in 1906. In the 1920s Dr. Louis Sauer developed a vaccine for whooping cough at Evanston Hospital (Chicago, IL). In 1925, the Danish physician Thorvald Madsen was the first to test a whole-cell pertussis vaccine on a wide scale.[5] He used the vaccine to control outbreaks in the Faroe Islands in the North Sea. In 1942, the American scientist Pearl Kendrick combined the whole-cell pertussis vaccine with diphtheria and tetanus toxoids to generate the first DTP combination vaccine. To minimize the frequent side effects caused by the pertussis component of the vaccine, the Japanese scientist Yuji Sato developed an acellular pertussis vaccine consisting of purified haemagglutinins (HAs: filamentous HA and leucocytosis-promoting-factor HA), which are secreted by B. pertussis into the culture medium. Sato's acellular pertussis vaccine was used in Japan since the autumn of 1981.[6] Later versions of the acellular pertussis vaccine used in other countries consisted of additional defined components of B. pertussis and were often part of the DTaP combination vaccine.
Current status of pertussis vaccines
Pertussis vaccines are highly effective, strongly recommended, and save many infant lives every year. Though the protection they offer lasts only a few years, they are given so that immunity lasts through childhood, the time of greatest exposure and greatest risk.[7] The immunizations are given in combination with tetanus, diphtheria, polio and haemophilus influenzae type B immunizations, at ages 2, 3, and 4 months, and a later booster at 3 years and 4 months or soon after (http://www.immunisation.nhs.uk/).
The short term effectiveness of the vaccines and the presence of B. pertussis infection in adults and adolescents who may transmit the bacteria to infants have caused many in the medical field to call for booster immunizations at later ages. Although Canada, France, the U.S. and Germany now have approved booster shots for adolescents, adults, or both, other countries adhere to the tradition of discontinuing pertussis vaccination after the age of seven, from concerns that there are side effects associated with the first available "whole-cell" pertussis immunizations that tended to increase with age. The whole-cell vaccine is still used in poor countries, since it is cheaper than the newer and safer acellular formulation.
As the immunity from infection or vaccination lasts only a few years, the discontinuation of booster vaccination in older persons caused the emergence of a large pool of older persons lacking immunity, followed by an increase of adult-onset pertussis that accelerated beginning in about 2004.[8] This burgeoning outbreak is predicted to increasingly infect adults and adolescents with debilitating cases, but poses even more serious public health dangers to newborns. As adolescent and adult cases surge, newborns are again at risk of exposure to pertussis circulating in adolescents or adults in the community before the infants' vaccinations can be completed.
The decision to resume vaccinating teens and adults reflects in part that the newer acellular vaccine, known as DTaP, has greatly reduced the incidence of adverse effects observed with the earlier "whole-cell" pertussis vaccine. An acellular vaccine preparation for adults and adolescents has been approved in Canada, Europe, and the United States. In the U.S., the Food and Drug Administration has authorized both the use of the vaccines Boostrix (GlaxoSmithKline) for 10-18 year olds in May 2005 and Adacel (Sanofi Pasteur) for 11-64 year olds in August 2005.[9] These vaccines are recommended for all teens and adults within the indicated age ranges, except for those with a history of adverse reaction to the whole-cell pertussis vaccines. The most serious side-effects of traditional "whole-cell" pertussis immunizations were neurological: and included seizures and hypotonic episodes.
Whole-cell pertussis vaccine controversy
Much of the controversy surrounding the DTP vaccine in the 1970s and 1980s related to the question of whether the whole-cell pertussis component caused permanent brain injury in rare cases. Although it was well-established that the pertussis component of the DTP vaccine accounted for most of the minor local and systemic side effects in many vaccinated infants, several published studies failed to show a causal relationship between administration of the DTP vaccine and permanent brain injury. However, criticism of these studies and well-publicized anecdotal reports of DTP-induced permanent disability and death gave rise to anti-DTP movements.[10] In addition, a number of children suffered allergic reactions to the pertussis vaccination, including severe seizures. Despite this, doctors recommended the vaccine, and even threatened to report parents who refused to vaccinate their children.
By the late 1970s, publicity about adverse reactions and deaths following pertussis vaccination caused the immunization rate to fall in several countries, including Great Britain, Sweden, and Japan. In many cases, a dramatic increase in the incidence of pertussis followed.[11] These developments led Yuji Sato to introduce a safer acellular version of the pertussis vaccine for Japan in 1981. Nevertheless, other countries continued to use the whole-cell DTP formulation.
In the United States, low profit margins and an increase in vaccine-related lawsuits led many manufacturers to stop producing the DTP vaccine by the early 1980s. In 1982, the television documentary "DTP: Vaccine Roulette" depicted the lives of children whose severe disabilities were blamed on the DTP vaccine. The negative publicity generated by the documentary led to a tremendous increase in the number of lawsuits filed against vaccine manufacturers.[12] By 1985, manufacturers of vaccines had difficulty obtaining liability insurance. The price of the DTP vaccine skyrocketed, leading to shortages around the country. Only one manufacturer of the DPT vaccine remained in the U.S. by the end of 1985. To avert a vaccine crisis, Congress in 1986 passed the National Childhood Vaccine Injury Act (NCVIA), which established a federal no-fault system to compensate victims of injury caused by mandated vaccines.[13] Since then, the prices of vaccines have stabilized, and the number of lawsuits filed against DTP manufacturers has dwindled. The majority of claims that have been filed through the NCVIA have been related to injuries allegedly caused by the whole-cell DTP vaccine. The acellular pertussis vaccine was approved in the United States in 1992 for use in the combination DTaP vaccine. Research has shown the acellular vaccine to be safe, with few reports of adverse effects.[14] Although the whole-cell DTP vaccine is no longer used in the United States, it is still purchased by the World Health Organization and distributed to developing nations because of its much reduced cost compared to the acellular DTaP vaccine.
Epidemiology
Before vaccines, an average of 157 cases per 100,000 persons were reported in the U.S., with peaks reported every two to five years; more than 93% of reported cases occurred in children under 10 years of age. The actual incidence was likely much higher. After vaccinations were introduced in the 1940s, incidence fell dramatically to less than 1 per 100,000 by 1970. Incidence rates have increased somewhat since 1980. Pertussis is the only vaccine-preventable disease that is associated with increasing deaths in the U.S. The number of deaths increased from 4 in 1996 to 17 in 2001, almost all of which were infants under one year.[15]
References
- ↑ Finger H, von Koenig CHW (1996). Bordetella–Clinical Manifestations. In: Barron's Medical Microbiology (Barron S et al, eds.) (4th ed. ed.). Univ of Texas Medical Branch. ISBN 0-9631172-1-1. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mmed.section.1694.
- ↑ Symptoms and Sounds from WhoopingCough.net
- ↑ Mattoo S, Cherry JD (2005). "Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies". Clin Microbiol Rev 18 (2): 326–82. doi:. PMID 15831828.
- ↑ http://files.dcp2.org/pdf/expressbooks/vaccine.pdf Vaccine-Preventable Diseases (Disease Control Priorities Project)Table 20.1, page 390 ©2006 The International Bank for Reconstruction and Development The World Bank 1818 H Street NW Washington DC 20433 Telephone: 202-473-1000 Internet: www.worldbank.org E-mail: feedback@worldbank.org
- ↑ Baker JP, Katz SL (2004). "Childhood vaccine development: an overview". Pediatr. Res. 55 (2): 347–56. doi:. PMID 14630981.
- ↑ Sato Y, Kimura M, Fukumi H (1984). "Development of a pertussis component vaccine in Japan". Lancet 1 (8369): 122–6. doi:. PMID 6140441.
- ↑ Versteegh FGA, Schellekens JFP, Fleer A, Roord JJ. (2005). "Pertussis: a concise historical review including diagnosis, incidence, clinical manifestations and the role of treatment and vaccination in management.". Rev Med Microbiol 16 (3): 79–89. http://www.revmedmicrobiol.com/pt/re/revmedmicrob/abstract.00013542-200508000-00001.htm.
- ↑ Enduring and Painful, Pertussis Leaps Back -- By KATE MURPHY New York Times -- February 22, 2005
- ↑ "Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed, ADACEL, Aventis Pasteur Ltd". Retrieved on 2006-05-01.
- ↑ Geier D, Geier M (2002). "The true story of pertussis vaccination: a sordid legacy?". Journal of the history of medicine and allied sciences 57 (3): 249–84. doi:. PMID 12211972.
- ↑ Gangarosa EJ, Galazka AM, Wolfe CR, Phillips LM, Gangarosa RE, Miller E, Chen RT (1998). "Impact of anti-vaccine movements on pertussis control: the untold story". Lancet 351 (9099): 356–61. doi:. PMID 9652634.
- ↑ Evans G (2006). "Update on vaccine liability in the United States: presentation at the National Vaccine Program Office Workshop on strengthening the supply of routinely recommended vaccines in the United States, 12 February 2002". Clin. Infect. Dis. 42 Suppl 3: S130–7. doi:. PMID 16447135.
- ↑ Smith MH (1988). "National Childhood Vaccine Injury Compensation Act". Pediatrics 82 (2): 264–9. PMID 3399300.
- ↑ Pichichero ME, Rennels MB, Edwards KM, et al (June 2005). "Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults". JAMA 293 (24): 3003–11. doi:. PMID 15933223.
- ↑ Gregory DS (2006). "Pertussis: a disease affecting all ages". Am Fam Physician 74 (3): 420–6. PMID 16913160. http://www.aafp.org/afp/20060801/420.html.
External links
- Pertussis at Todar's Online Textbook of Bacteriology
- Pertussis: new insights in diagnosis, incidence and clinical manifestations, F.G.A. Versteegh, Thesis, 2005
- Symptoms and Sounds
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||