Polymer
A polymer is a large molecule (macromolecule) composed of repeating structural units typically connected by covalent chemical bonds. While polymer in popular usage suggests plastic, the term actually refers to a large class of natural and synthetic materials with a variety of properties and purposes.
| Polypropylene | |
|---|---|
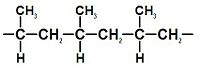 |
|
| IUPAC name | poly(propene) |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Well-known examples of polymers include plastics and proteins. A simple example is polypropylene, whose repeating unit structure is shown at the right. However, polymers are not just limited to having predominantly carbon backbones, elements such as silicon form familiar materials such as silicones, examples being silly putty and waterproof plumbing sealant. The backbone of DNA is in fact based on a phosphodiester bond.
Natural polymer materials such as shellac and amber have been in use for centuries. Biopolymers such as proteins and nucleic acids play crucial roles in biological processes. A variety of other natural polymers exist, such as cellulose, which is the main constituent of wood and paper.
The list of synthetic polymers includes Bakelite, neoprene, nylon, PVC, polystyrene, polyacrylonitrile, PVB, silicone, and many more.
Polymers are studied in the fields of polymer chemistry, polymer physics, and polymer science.
Functionality
This characteristic of a monomer helps in deciding whether a particular monomer can form a polymer or not. [1] It is actually defined as the number of reaction sites present around the monomer in order to help in forming chemical covalent bonds,so that it can form a polymer.
The basic required functionality is 2.
Etymology
The word polymer is derived from the Greek words πολυ (poly), meaning "many"; and μέρος (meros), meaning "part". The term was coined in 1833 by Jöns Jakob Berzelius, although his definition of a polymer was quite different from the modern definition. (see Jöns Jakob Berzelius#New chemical terms)
Historical development
Starting in 1811, Henri Braconnot did pioneering work in derivative cellulose compounds, perhaps the earliest important work in polymer science. The development of vulcanization later in the nineteenth century improved the durability of the natural polymer rubber, signifying the first popularized semi-synthetic polymer. In 1907, Leo Baekeland created the first completely synthetic polymer, Bakelite, by reacting phenol and formaldehyde at precisely controlled temperature and pressure. Bakelite was then publicly introduced in 1909.
Despite significant advances in synthesis and characterization of polymers, a correct understanding of polymer molecular structure did not emerge until the 1920s. Before that, scientists believed that polymers were clusters of small molecules (called colloids), without definite molecular weights, held together by an unknown force, a concept known as association theory. In 1922, Hermann Staudinger proposed that polymers consisted of long chains of atoms held together by covalent bonds, an idea which did not gain wide acceptance for over a decade and for which Staudinger was ultimately awarded the Nobel Prize. Work by Wallace Carothers in the 1920s also demonstrated that polymers could be synthesized rationally from their constituent monomers. An important contribution to synthetic polymer science was made by the Italian chemist Giulio Natta and the German chemist Karl Ziegler, who won the Nobel Prize in Chemistry in 1963 for the development of the Ziegler-Natta catalyst. Further recognition of the importance of polymers came with the award of the Nobel Prize in Chemistry in 1974 to Paul Flory, whose extensive work on polymers included the kinetics of step-growth polymerization and of addition polymerization, chain transfer, excluded volume, the Flory-Huggins solution theory, and the Flory convention.
Synthetic polymer materials such as nylon, polyethylene, Teflon, and silicone have formed the basis for a burgeoning polymer industry. These years have also shown significant developments in rational polymer synthesis. Most commercially important polymers today are entirely synthetic and produced in high volume on appropriately scaled organic synthetic techniques. Synthetic polymers today find application in nearly every industry and area of life. Polymers are widely used as adhesives and lubricants, as well as structural components for products ranging from children's toys to aircraft. They have been employed in a variety of biomedical applications ranging from implantable devices to controlled drug delivery. Polymers such as poly (methyl methacrylate) find application as photoresist materials used in semiconductor manufacturing and low-k dielectrics for use in high-performance microprocessors. Recently, polymers have also been employed in the development of flexible polymer-based substrates for electronic displays.
Polymer synthesis
Polymerization is the process of combining many small molecules known as monomers into a covalently bonded chain. During the polymerization process, some chemical groups may be lost from each monomer. The distinct piece of each monomer that is incorporated into the polymer is known as a repeat unit or monomer residue.
Laboratory synthesis
Laboratory synthetic methods are generally divided into two categories, condensation polymerization and addition polymerization. However, some newer methods such as plasma polymerization do not fit neatly into either category. Synthetic polymerization reactions may be carried out with or without a catalyst. Efforts towards rational synthesis of biopolymers via laboratory synthetic methods, especially artificial synthesis of proteins, is an area of intense research.
Biological synthesis
There are three main classes of biopolymers: polysaccharides, polypeptides, and polynucleotides. In living cells, they may be synthesized by enzyme-mediated processes, such as the formation of DNA catalyzed by DNA polymerase. The synthesis of proteins involves multiple enzyme-mediated processes to transcribe genetic information from the DNA and subsequently translate that information to synthesize the specified protein from amino acids. The protein may be modified further following translation in order to provide appropriate structure and functioning.
Modification of natural polymers
Many commercially important polymers are synthesized by chemical modification of naturally occurring polymers. Prominent examples include the reaction of nitric acid and cellulose to form nitrocellulose and the formation of vulcanized rubber by heating natural rubber in the presence of sulphur.
Polymer structure
The structural properties of a polymer relate to the physical arrangement of monomer residues along the backbone of the chain. Structure has a strong influence on the other properties of a polymer. For example, a linear chain polymer may be soluble or insoluble in water depending on whether it is composed of polar monomers (such as ethylene oxide) or nonpolar monomers (such as styrene). On the other hand, two samples of natural rubber may exhibit different durability, even though their molecules comprise the same monomers. Polymer scientists have developed terminology to describe precisely both the nature of the monomers as well as their relative arrangement.
Monomer identity
The identity of the monomers comprising the polymer is generally the first and most important attribute of a polymer. The repeat unit is the constantly repeated unit of the chain and is also characteristic of the polymer. Polymer nomenclature is generally based upon the type of monomers comprising the polymer. Polymers that contain only a single type of monomer are known as homopolymers, while polymers containing a mixture of monomers are known as copolymers. Poly(styrene), for example, is composed only of styrene monomers, and is therefore classified as a homopolymer. Ethylene-vinyl acetate, on the other hand, contains more than one variety of monomer and is thus a copolymer. Some biological polymers are composed of a variety of different but structurally related monomers, such as polynucleotides composed of nucleotide subunits.
The repeating unit of the polymer may be different from the starting monomer(s), for example in condensation polymerization. A simple example is PET polyester. The monomers are terephthalic acid (HOOC-C6H4-COOH) and ethylene glycol (HO-CH2-CH2-OH) but the repeating unit is -OC-C6H4-COO-CH2-CH2-O-, which corresponds to the combination of the two monomers with the loss of two water molecules.
A polymer molecule containing ionizable subunits is known as a polyelectrolyte. An ionomer is a subclass of polyelectrolyte with a low fraction of ionizable subunit.
Tacticity
Tacticity describes the relative stereochemistry of chiral centers in neighboring structural units within a macromolecule. There are three types: isotactic, atactic, and syndiotactic.
Chain linearity

The simplest form of polymer molecule is a straight chain or linear' polymer, composed of a single main chain. The flexibility of an unbranched chain polymer is characterized by its persistence length. A branched polymer molecule is composed of a main chain with one or more substituent side chains or branches. Special types of branched polymers include star polymers, comb polymers, and brush polymers. If the polymer contains a side chain that has a different composition or configuration than the main chain, the polymer is called a graft or grafted polymer. A cross-link suggests a branch point from which four or more distinct chains emanate. A polymer molecule with a high degree of crosslinking is referred to as a polymer network.[3] Sufficiently high crosslink concentrations may lead to the formation of an infinite network, also known as a gel, in which networks of chains are of unlimited extent — essentially all chains have linked into one molecule.[4]
Chain length
Polymer bulk properties may be strongly dependent on the size of the polymer chain. Like any molecule, a polymer molecule's size may be described in terms of molecular weight or mass. In polymers, however, the molecular mass may be expressed in terms of degree of polymerization, essentially the number of monomer units which comprise the polymer. For synthetic polymers, the molecular weight is expressed statistically to describe the distribution of molecular weights in the sample. This is because of the fact that almost all industrial processes produce a distribution of polymer chain sizes. Examples of such statistics include the number average molecular weight and weight average molecular weight. The ratio of these two values is the polydispersity index, commonly used to express the "width" of the molecular weight distribution.
The maximum length of a polymer chain is its contour length.
Monomer arrangement in copolymers
Monomers within a copolymer may be organized along the backbone in a variety of ways.
- Alternating copolymers possess regularly alternating monomer residues.
- Periodic copolymers have monomer residue types arranged in a repeating sequence.
- Random copolymers have a random sequence of monomer residue types.
- Statistical copolymers have monomer residues arranged according to a known statistical rule.
- Block copolymers have two or more homopolymer subunits linked by covalent bonds. Block copolymers with two or three distinct blocks are called diblock copolymers and triblock copolymers, respectively.
Polymer properties
Types of polymer properties can be broadly divided into several categories based upon scale. At the nano-micro scale there are properties that directly describe the chain itself, and can be thought of as polymer structure. At an intermediate mesoscopic level there are properties that describe the morphology of the polymer matrix in space. At the macroscopic level properties describe the bulk behavior of the polymer.
The bulk properties of a polymer are those most often of end-use interest. These are the properties that dictate how the polymer actually behaves on a macroscopic scale.
Relationship between chain length and polymer properties
Polymer bulk properties are strongly dependent upon their structure and mesoscopic behavior. A number of qualitative relationships between structure and properties are known.
Increasing chain length tends to decrease chain mobility, increase strength and toughness, and increase the glass transition temperature (Tg). This is a result of the increase in chain interactions such as Van der Waals attractions and entanglements that come with increased chain length. These interactions tend to fix the individual chains more strongly in position and resist deformations and matrix breakup, both at higher stresses and higher temperatures. Chain length is related to melt viscosity roughly as 1:103.2, so that a tenfold increase in polymer chain length results in a viscosity increase of over 1000 times.
Crystallinity
When applied to polymers, the term crystalline has a somewhat ambiguous usage. In some cases, the term crystalline finds identical usage to that used in conventional crystallography. For example, the structure of a crystalline protein or polynucleotide, such as a sample prepared for x-ray crystallography, may be defined in terms of a conventional unit cell composed of one or more polymer molecules with cell dimensions of hundreds of angstroms or more.
A synthetic polymer may be lightly described as crystalline if it contains regions of three-dimensional ordering on atomic (rather than macromolecular) length scales, usually arising from intramolecular folding and/or stacking of adjacent chains. Synthetic polymers may consist of both crystalline and amorphous regions; the degree of crystallinity may be expressed in terms of a weight fraction or volume fraction of crystalline material. Few synthetic polymers are entirely crystalline.[5]
The crystallinity of polymers is characterized by their degree of crystallinity, ranging from zero for a completely noncrystalline polymer to one for a theoretical completely crystalline polymer. Increasing degree of crystallinity tends to make a polymer more rigid. It can also lead to greater brittleness. Polymers with a degree of crystallinity approaching zero or one will tend to be transparent, while polymers with intermediate degrees of crystallinity will tend to be opaque due to light scattering by crystalline or glassy regions.
Tensile strength
The tensile strength of a material quantifies how much stress the material will endure before failing. [6] [7] This is very important in applications that rely upon a polymer's physical strength or durability. For example, a rubber band with a higher tensile strength will hold a greater weight before snapping. In general tensile strength increases with polymer chain length.
Young's modulus of elasticity
Young's Modulus quantifies the elasticity of the polymer. It is defined, for small strains, as the ratio of rate of change of stress to strain. Like tensile strength, this is highly relevant in polymer applications involving the physical properties of polymers, such as rubber bands. The modulus is strongly dependent on temperature.
Transport properties
Transport properties such as diffusivity relate to how rapidly molecules move through the polymer matrix. These are very important in many applications of polymers for films and membranes.
Melting point
The term melting point, when applied to polymers, suggests not a solid-liquid phase transition but a transition from a crystalline or semi-crystalline phase to a solid amorphous phase. Though abbreviated as simply Tm, the property in question is more properly called the crystalline melting temperature. Among synthetic polymers, crystalline melting is only discussed with regards to thermoplastics, as thermosetting polymers will decompose at high temperatures rather than melt.
Boiling point
The boiling point of a polymer substance is never defined because polymers will decompose before reaching theoretical boiling temperatures.
Glass transition temperature
A parameter of particular interest in synthetic polymer manufacturing is the glass transition temperature (Tg), which describes the temperature at which amorphous polymers undergo a second-order phase transition from a rubbery, viscous amorphous solid to a brittle, glassy amorphous solid. The glass transition temperature may be engineered by altering the degree of branching or crosslinking in the polymer or by the addition of plasticizer.[8]
Mixing behavior
In general, polymeric mixtures are far less miscible than mixtures of small molecule materials. This effect is a result of the fact that the driving force for mixing is usually entropics, not energetics. In other words, miscible materials usually form a solution not because their interaction with each other is more favorable than their self-interaction, but because of an increase in entropy and hence free energy associated with increasing the amount of volume available to each component. This increase in entropy scales with the number of particles (or moles) being mixed. Since polymeric molecules are much larger and hence generally have much higher specific volumes than small molecules, the number of molecules involved in a polymeric mixture are far less than the number in a small molecule mixture of equal volume. The energetics of mixing, on the other hand, are comparable on a per volume basis for polymeric and small molecule mixtures. This tends to increase the free energy of mixing for polymer solutions and thus make solvation less favorable. Thus, concentrated solutions of polymers are far rarer than those of small molecules.
In dilute solution, the properties of the polymer are characterized by the interaction between the solvent and the polymer. In a good solvent, the polymer appears swollen and occupies a large volume. In this scenario, intermolecular forces between the solvent and monomer subunits dominate over intramolecular interactions. In a bad solvent or poor solvent, intramolecular forces dominate and the chain contracts. In the theta solvent, or the state of the polymer solution where the value of the second virial coefficient becomes 0, the intermolecular polymer-solvent repulsion balances exactly the intramolecular monomer-monomer attraction. Under the theta condition (also called the Flory condition), the polymer behaves like an ideal random coil.
Chain conformation
The space occupied by a polymer molecule is generally expressed in terms of radius of gyration, which is an average distance from the center of mass of the chain to the chain itself. Alternatively, it may be expressed in terms of pervaded volume, which is the volume of solution spanned by the polymer chain and scales with the cube of the radius of gyration[9].
Branching
Branching of polymer chains also affect the bulk properties of polymers. Long chain branches may increase polymer strength, toughness, and Tg due to an increase in the number of entanglements per chain. Random length and atactic short chains, on the other hand, may reduce polymer strength due to disruption of organization. Short side chains may likewise reduce crystallinity due to disruption of the crystal structure. Reduced crystallinity may also be associated with increased transparency due to light scattering by small crystalline regions. A good example of this effect is related to the range of physical attributes of polyethylene. High-density polyethylene (HDPE) has a very low degree of branching, is quite stiff, and is used in applications such as milk jugs. Low-density polyethylene (LDPE), on the other hand, has significant numbers of short branches, is quite flexible, and is used in applications such as plastic films. The branching index of the polymer is a parameter that characterizes the effect of long-chain branches on the size of a branched macromolecule in solution. Dendrimers are a special case of polymer where every monomer unit is branched. This tends to reduce intermolecular chain entanglement and crystallization. Alternatively, dendritic polymers are not perfectly branched but share similar properties to dendrimers due to their high degree of branching.
Chemical crosslinking
Crosslinking tends to increase Tg and increase strength and toughness. Crosslinking consists of the formation of chemical bonds between chains. Among other applications, this process is used to strengthen rubbers in a process known as vulcanization, which is based on crosslinking by sulphur. Car tires, for example, are highly crosslinked in order to reduce the leaking of air out of the tire and to toughen their durability. Eraser rubber, on the other hand, is not crosslinked to allow flaking of the rubber and prevent damage to the paper.
Inclusion of plasticizers
Inclusion of plasticizers tends to lower Tg and increase polymer flexibility. Plasticizers are generally small molecules that are chemically similar to the polymer and create gaps between polymer chains for greater mobility and reduced interchain interactions. A good example of the action of plasticizers is related to polyvinylchlorides or PVCs. A uPVC, or unplasticized polyvinylchloride, is used for things such as pipes. A pipe has no plasticizers in it, because it needs to remain strong and heat-resistant. Plasticized PVC is used for clothing for a flexible quality. Plasticizers are also put in some types of cling film to make the polymer more flexible.
Standardized polymer nomenclature
There are multiple conventions for naming polymer substances. Many commonly used polymers, such as those found in consumer products, are referred to by a common or trivial name. The trivial name is assigned based on historical precedent or popular usage rather than a standardized naming convention. Both the American Chemical Society[10] and IUPAC[11] have proposed standardized naming conventions; the ACS and IUPAC conventions are similar but not identical. [12] Examples of the differences between the various naming conventions are given in the table below:
| Common Name | ACS Name | IUPAC Name |
|---|---|---|
| Poly (ethylene oxide) or (PEO) | poly(oxyethylene) | poly(oxyethene) |
| Poly (ethylene terephthalate) or (PET) | poly (oxy-1,2-ethanediyloxycarbonyl -1,4-phenylenecarbonyl) | poly (oxyetheneoxyterephth= aloyl) |
| Nylon | poly[amino(1-oxo-1,6-hexanediyl)] | poly[amino(1-oxohexan-1,6-diyl)] |
In both standardized conventions, the polymers' names are intended to reflect the monomer(s) from which they are synthesized rather than the precise nature of the repeating subunit. For example, the polymer synthesized from the simple alkene ethene is called polyethylene, retaining the -ene suffix even though the double bond is removed during the polymerization process:
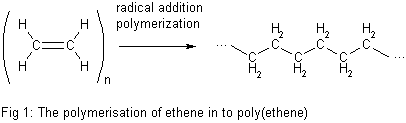
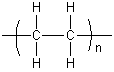
Chemical properties of polymers
The attractive forces between polymer chains play a large part in determining a polymer's properties. Because polymer chains are so long, these interchain forces are amplified far beyond the attractions between conventional molecules. Different side groups on the polymer can lend the polymer to ionic bonding or hydrogen bonding between its own chains. These stronger forces typically result in higher tensile strength and melting points.
The intermolecular forces in polymers can be affected by dipoles in the monomer units. Polymers containing amide or carbonyl groups can form hydrogen bonds between adjacent chains; the partially positively charged hydrogen atoms in N-H groups of one chain are strongly attracted to the partially negatively charged oxygen atoms in C=O groups on another. These strong hydrogen bonds, for example, result in the high tensile strength and melting point of polymers containing urethane or urea linkages. Polyesters have dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogen atoms in H-C groups. Dipole bonding is not as strong as hydrogen bonding, so a polyester's melting point and strength are lower than Kevlar's (Twaron), but polyesters have greater flexibility.
Ethene, however, has no permanent dipole. The attractive forces between polyethylene chains arise from weak van der Waals forces. Molecules can be thought of as being surrounded by a cloud of negative electrons. As two polymer chains approach, their electron clouds repel one another. This has the effect of lowering the electron density on one side of a polymer chain, creating a slight positive dipole on this side. This charge is enough to attract the second polymer chain. Van der Waals forces are quite weak, however, so polyethene can have a lower melting temperature compared to other polymers.
Polymer characterization
The characterization of a polymer requires several parameters which need to be specified. This is because a polymer actually consists of a statistical distribution of chains of varying lengths, and each chain consists of monomer residues which affect its properties.
A variety of lab techniques are used to determine the properties of polymers. Techniques such as wide angle X-ray scattering, small angle X-ray scattering, and small angle neutron scattering are used to determine the crystalline structure of polymers. Gel permeation chromatography is used to determine the number average molecular weight, weight average molecular weight, and polydispersity. FTIR, Raman and NMR can be used to determine composition. Thermal properties such as the glass transition temperature and melting point can be determined by differential scanning calorimetry and dynamic mechanical analysis. Pyrolysis followed by analysis of the fragments is one more technique for determining the possible structure of the polymer. Thermogravimetry is a useful technique to evaluate the thermal stability of the polymer. Detailed analyses of TG curves also allow us to know a bit of the phase segregation in polymers.
Polymer degradation
Polymer degradation is a change in the properties—tensile strength, colour, shape, etc.—of a polymer or polymer-based product under the influence of one or more environmental factors, such as heat, light or chemicals. It is often due to the hydrolysis of the bonds connecting the polymer chain, which in turn leads to a decrease in the molecular mass of the polymer. These changes may be undesirable, such as changes during use, or desirable, as in biodegradation or deliberately lowering the molecular mass of a polymer. Such changes occur primarily because of the effect of these factors on the chemical composition of the polymer. Ozone cracking and UV degradation are specific failure modes for certain polymers.
The degradation of polymers to form smaller molecules may proceed by random scission or specific scission. The degradation of polyethylene occurs by random scission—a random breakage of the linkages (bonds) that hold the atoms of the polymer together. When heated above 450°C it degrades to form a mixture of hydrocarbons. Other polymers—like polyalphamethylstyrene—undergo specific chain scission with breakage occurring only at the ends. They literally unzip or depolymerize to become the constituent monomer.
However, the degradation process can be useful from the viewpoints of understanding the structure of a polymer or recycling/reusing the polymer waste to prevent or reduce environmental pollution. Polylactic acid and polyglycolic acid, for example, are two polymers that are useful for their ability to degrade under aqueous conditions. A copolymer of these polymers is used for biomedical applications, such as hydrolysable stitches that degrade over time after they are applied to a wound. These materials can also be used for plastics that will degrade over time after they are used and will therefore not remain as litter.
Product failure

In a finished product, such a change is to be prevented or delayed. Failure of safety-critical polymer components can cause serious accidents, such as fire in the case of cracked and degraded polymer fuel lines. Chlorine-induced cracking of acetal resin plumbing joints and polybutylene pipes has caused many serious floods in domestic properties, especially in the USA in the 1990s. Traces of chlorine in the water supply attacked vulnerable polymers in the plastic plumbing, a problem which occurs faster if any of the parts have been poorly extruded or injection moulded. Attack of the acetal joint occurred because of faulty moulding leading to cracking along the threads of the fitting, which are serious stress concentrations.

Polymer oxidation leads to cracking and failure of the parts affected and has caused accidents involving medical devices. One of the oldest known failure modes is ozone cracking caused by chain scission when ozone gas attacks susceptible elastomers such as natural rubber and nitrile rubber. They possess double bonds in their repeat units which are cleaved during ozonolysis. Cracks in fuel lines can penetrate the bore of the tube and cause fuel leakage. If cracking occurs in the engine compartment, electric sparks can ignite the gasoline and can cause a serious fire.
Fuel lines can also be attacked by another form of degradation: hydrolysis. Nylon 6,6 is susceptible to acid hydrolysis, and in one accident, a fractured fuel line led to a spillage of diesel into the road. If diesel fuel leaks onto the road, accidents to following cars can be caused by the slippery nature of the deposit, which is like black ice.
References
- ↑ Campbell, Neil A.; Brad Williamson; Robin J. Heyden (2006). Biology: Exploring Life. Boston, Massachusetts: Pearson Prentice Hall. ISBN 0-13-250882-6. http://www.phschool.com/el_marketing.html.
- ↑ Y. Roiter and S. Minko, AFM Single Molecule Experiments at the Solid-Liquid Interface: In Situ Conformation of Adsorbed Flexible Polyelectrolyte Chains, Journal of the American Chemical Society, vol. 127, iss. 45, pp. 15688-15689 (2005)
- ↑ IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.
- ↑ Painter, P and Coleman, M. "Fundamentals of Polymer Science". 1997, 96-100.
- ↑ IUPAC Purple Book: Definition of terms relating to crystalline polymers (1988) See Sec.1.3 Degree of Crystallinity
- ↑ Ashby, Michael and Jones, David. Engineering Materials. p. 191-195. Oxford: Butterworth-Heinermann, 1996. Ed. 2.
- ↑ Meyers and Chawla. Mechanical Behavior of Materials. pg. 41. Prentice Hall, Inc. 1999.
- ↑ Brandrup, J.; Immergut, E.H.; Grulke, E.A.; eds Polymer Handbook 4th Ed. New York: Wiley-Interscience, 1999.
- ↑ Rubinstein, M and Colby, R. "Polymer Physics". 2003, 13.
- ↑ CAS: Index Guide, Appendix IV (© 1998).
- ↑ IUPAC. "Nomenclature of Regular Single-Strand Organic Polymers". Pure Appl. Chem. 1976, 48, 373-385.
- ↑ Macromolecular Nomenclature Note No. 18
Bibliography
- Allcock, Harry R.; Lampe, Frederick W.; and Mark, James E. Contemporary Polymer Chemistry, Pearson Education, 3rd edition (2003).
- Cowie, J.M.G. Polymers: Chemistry and Physics of Modern Materials, Blackie (in USA: Chapman and Hall), 2nd edition (1991).
- Ezrin, Meyer. Plastics Failure Guide: Cause and Prevention, Hanser-SPE (1996).
- Lewis, Peter Rhys; Reynolds, K.; and Gagg, C. Forensic Materials Engineering: Case studies, CRC Press (2004).
- Wright, David C. Environmental Stress Cracking of Plastics, RAPRA (2001).
See also
- Biopolymer
- Copolymer
- Electroactive polymers
- Forensic polymer engineering
- Glass transition temperature
- Important publications in polymer chemistry
- Monomer
- Nurdle
- Polyanhydrides
- Polymerization
- Polymer classes
- Polymer degradation
- Polymer engineering
- Polymer science
- Polymersome
- Shape memory polymer
- Smart materials