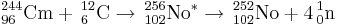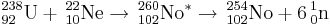Nobelium
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | nobelium, No, 102 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | actinides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | n/a, 7, f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | unknown, probably metallic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | [259] g·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 8, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 641.6 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1254.3 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 2605.1 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 10028-14-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most-stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nobelium (pronounced /noʊˈbɛliəm/ or /noʊˈbiːliəm/) is a synthetic element with the symbol No and atomic number 102.
It was first correctly identified in 1956 by scientists at the Flerov Laboratory of Nuclear Reactions in Dubna, Russia. Little is known about the element but limited chemical experiments have shown that it forms a stable divalent ion in solution as well as the predicted trivalent ion that is associated with its presence as one of the actinoids.
Contents |
Discovery profile
The discovery of element 102 was first announced by physicists at the Nobel Institute in Sweden in 1957. The team reported that they created an isotope with a half-life of 10 minutes, decaying by emission of an 8.5 MeV alpha particle, after bombarding 244Cm with 13C nuclei. The activity was assigned to 251102 or 253102. The scientists proposed the name nobelium (No) for the new element. Later they retracted their claim and associated the activity to background effects.
The synthesis of element 102 was then claimed in April 1958 at the University of California, Berkeley by Albert Ghiorso, Glenn T. Seaborg, John R. Walton and Torbjørn Sikkeland. The team used the new heavy-ion linear accelerator (HILAC) to bombard a curium target (95% 244Cm and 5% 246Cm) with 13C and 12C ions. They were unable to confirm the 8.5 MeV activity claimed by the Swedes but were instead able to detect decays from 250Fm, supposedly the daughter of 254102, which had an apparent half-life of ~3 s. In 1959 the team continued their studies and claimed that they were able to produce an isotope that decayed predominantly by emission of an 8.3 MeV alpha particle, with a half-life of 3 s with an associated 30% spontaneous fission branch. The activity was initially assigned to 254No but later changed to 252No. The Berkeley team decided to adopt the name nobelium for the element.
Further work in 1961 on the attempted synthesis of element 103 (see lawrencium) produced evidence for a Z=102 alpha activity decaying by emission of an 8.2 MeV particle with a half-life of 15 s, and assigned to 255No.
Following initial work between 1958-1964, in 1966, a team at the Flerov Laboratory of Nuclear Reactions (FLNR) reported that they had been able to detect 250Fm from the decay of a parent nucleus (254No) with a half-life of ~50s, in contradiction to the Berkeley claim. Furthermore, they were able to show that the parent decayed by emission of 8.1 MeV alpha particles with a half-life of ~35 s.
In 1969, the Dubna team carried out chemical experiments on element 102 and concluded that it behaved as the heavier homologue of Ytterbium. The Russian scientists proposed the name joliotium (Jo) for the new element.
Later work in 1967 at Berkeley and 1971 at Oak Ridge fully confirmed the discovery of element 102 and clarified earlier observations.
In 1992, the IUPAC-IUPAP Transfermium Working Group (TWG) assessed the claims of discovery and concluded that only the Dubna work from 1966 correctly detected and assigned decays to Z=102 nuclei at the time. The Dubna team are therefore officially recognised as the discoverers of nobelium although it is possible that it was detected at Berkeley in 1959.
Naming
Element 102 was first named nobelium (No) by its claimed discoverers in 1957 by scientists at the Nobel Institute in Sweden. The name was later adopted by Berkeley scientists who claimed its discovery in 1959.
The International Union of Pure and Applied Chemistry (IUPAC) officially recognised the name nobelium following the Berkeley results.
In 1969, Russian scientists working in Dubna disputed the claims of these groups and suggested the name joliotium (Jo), in recognition of the work of Frédéric Joliot-Curie.
In 1992, the TWG recognised the Dubna scientists as the official discoverers and acknowledged that the adoption of nobelium as the official name had been made prematurely.
Subsequently, there are indications that the IUPAC suggested the name flerovium (Fl) for the element in recognition of the Dubna laboratory and the name has been used in the literature in reference to the element.
However, in 1994, and subsequently in 1997, the IUPAC ratified the name nobelium (No) for the element on the basis that it had become entrenched in the literature over the course of 30 years and that Alfred Nobel should be commemorated in this fashion.
Contrary to some suggestions, at no time has element 102 been referred to as unnilbium due to the above circumstances.
Electronic structure
Nobelium is element 102 in the Periodic Table. The two forms of the projected electronic structure are:
Bohr model: 2, 8, 18, 32, 32, 8, 2
Quantum mechanical model: 1s22s22p63s23p64s23d10 4p65s24d105p66s24f145d10 6p67s25f14
Physical properties
The appearance of this element is unknown, however it is most likely silvery-white or gray and metallic. If sufficient amounts of nobelium were produced, it would pose a radiation hazard. Some sources quote a melting point of 827oC for nobelium but this cannot be substantiated from an official source and seems implausible regarding the requirements of such a measurement. However, the 1st, 2nd and 3rd ionization energies have been measured. In addition, an electronegativity value of 1.3 is also sometimes quoted. This is most definitely only an estimate since a true value can only be determined using a chemical compound of the element and no such compounds exist for nobelium.
Experimental chemistry
Aqueous phase chemistry
First experiments involving nobelium assumed that it predominantly formed a +III state like earlier actinoids. However, it was later found that nobelium forms a stable +II state in solution, although it can be oxidised to an oxidising +III state. A reduction potential of -1.78 V has been measured for the No3+ ion. The hexaaquanobelium(II) ion has been determined to have an ionic radius of 110 pm.
Summary of compounds and (complex) ions
| Formula | Names(s) |
|---|---|
| [No(H2O)6]3+ | hexaaquanobelium(III) |
| [No(H2O)6]2+ | hexaaquanobelium(II) |
Isotopes
Seventeen radioisotopes of nobelium have been characterized, with the most stable being 259No with a half-life of 58 minutes. Longer half-lives are expected for the as-yet-unknown 261No and 263No. An isomeric level has been found in 253No and K-isomers have been found in 250No, 252No and 254No to date.
Synthesis of isotopes as decay products
Isotopes of nobelium have also been identified in the decay of heavier elements. Observations to date are summarised in the table below:
| Evaporation Residue | Observed No isotope |
|---|---|
| 262Lr | 262No |
| 269Hs, 265Sg, 261Rf | 257No |
| 267Hs, 263Sg, 259Rf | 255No |
| 254Lr | 254No |
| 261Sg, 257Rf | 253No |
| 264Hs, 260Sg, 256Rf | 252No |
| 255Rf | 251No |
Chronology of isotope discovery
| Isotope | Year discovered | Discovery reaction |
|---|---|---|
| 250Nom | 2001 | 204Pb(48Ca,2n) |
| 250Nog | 2006 | 204Pb(48Ca,2n) |
| 251No | 1967 | 244Cm(12C,5n) |
| 252Nog | 1959 | 244Cm(12C,4n) |
| 252Nom | ~2002 | 206Pb(48Ca,2n) |
| 253Nog | 1967 | 242Pu(16O,5n),239Pu(18O,4n) |
| 253Nom | 1971 | 249Cf(12C,4n)[1] |
| 254Nog | 1966 | 243Am(15N,4n) |
| 254Nom1 | 1967? | 246Cm(13C,5n),246Cm(12C,4n) |
| 254Nom2 | ~2003 | 208Pb(48Ca,2n) |
| 255No | 1967 | 246Cm(13C,4n),248Cm(12C,5n) |
| 256No | 1967 | 248Cm(12C,4n),248Cm(13C,5n) |
| 257No | 1961? , 1967 | 248Cm(13C,4n) |
| 258No | 1967 | 248Cm(13C,3n) |
| 259No | 1973 | 248Cm(18O,α3n) |
| 260No | ? | 254Es + 22Ne,18O,13C - transfer |
| 261No | unknown | |
| 262No | 1988 | 254Es + 22Ne - transfer (EC of 262Lr) |
Isomerism in nobelium nuclides
254No The study of K-isomerism was recently studied by physicists at the University of Jyväskylä physics laboratory (JYFL). They were able to confirm a previously reported K-isomer and detected a second K-isomer. They assigned spins and parities of 8- and 16+ to the two K-isomers.
253No In 1971, Bemis et al. was able to determine an isomeric level decaying with a half-life of 31 µs from the decay of 257Rf. This was confirmed in 2003 at the GSI by also studying the decay of 257Rf. Further support in the same year from the FLNR appeared with a slightly higher half-life of 43.5 µs, decaying by M2 gamma emission to the ground state.
252No In a recent study by the GSI into K-isomerism in even-even isotopes, a K-isomer with a half-life of 110 ms was detected for 252No. A spin and parity of 8- was assigned to the isomer.
250No In 2003, scientists at the FLNR reported that they had been able to synthesise 249No which decayed by SF with a half-life of 54µs. Further work in 2006 by scientists at the ANL showed that the activity was actually due to a K-isomer in 250No. The ground state isomer was also detected with a very short half-life of 3.7µs.
Chemical yields of isotopes
Cold fusion
The table below provides cross-sections and excitation energies for cold fusion reactions producing nobelium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 1n | 2n | 3n | 4n |
|---|---|---|---|---|---|---|
| 48Ca | 208Pb | 256No | 254No: 2050 nb ; 22.3 MeV | |||
| 48Ca | 207Pb | 255No | 253No: 1310 nb ; 22.4 MeV | |||
| 48Ca | 206Pb | 254No | 253No: 58 nb ; 23.6 MeV | 252No: 515 nb ; 23.3 MeV | 251No: 30 nb ; 30.7 MeV | 250No: 260 pb ; 43.9 MeV |
| 48Ca | 204Pb | 252No | 250No:13.2 nb ; 23.2 MeV |
Hot fusion
The table below provides cross-sections and excitation energies for hot fusion reactions producing nobelium isotopes directly. Data in bold represents maxima derived from excitation function measurements. + represents an observed exit channel.
| Projectile | Target | CN | 3n | 4n | 5n | 6n |
|---|---|---|---|---|---|---|
| 26Mg | 232Th | 258No | 254No:1.6 nb | 253No:9 nb | 252No:8 nb | |
| 22Ne | 238U | 260No | 256No:40 nb | 255No:200 nb | 254No:15 nb | |
| 22Ne | 236U | 258No | 254No:7 nb | 253No:25 nb | 252No:15 nb |
Retracted isotopes
In 2003, scientists at the FLNR claimed to have discovered the lightest known isotope of nobelium. However, subsequent work showed that the 54 µs activity was actually due to 250No and the isotope 249No was retracted.
References
- ↑ see rutherfordium
Notes
- Los Alamos National Laboratory - Nobelium
- Guide to the Elements - Revised Edition, Albert Stwertka, (Oxford University Press; 1998) ISBN 0-19-508083-1
- It's Elemental - Nobelium
External links
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||