Nitrous oxide
| Nitrous oxide | |
|---|---|
 |
|
 |
|
| Identifiers | |
| CAS number | 10024-97-2 |
| PubChem | |
| ATC code | N01 |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | N2O |
| Molar mass | 44.0128 g/mol |
| Appearance | colorless gas |
| Density | 1222.8 kg m-3 (liquid) 1.8 kg m-3 (gas STP) |
| Melting point |
-90.86 °C, 182 K, -132 °F |
| Boiling point |
-88.48 °C, 185 K, -127 °F |
| Structure | |
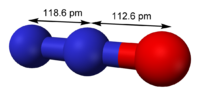
| Molecular shape | linear |
| Dipole moment | 0.166D |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
+82.05 |
| Pharmacology | |
| Routes of administration |
Inhalation |
| Metabolism | 0.004% |
| Elimination half-life |
5 minutes |
| Excretion | Respiratory |
| Legal status |
{{{legal_status}}} |
| Hazards | |
| NFPA 704 |
 0
2
0
OX
|
| R-phrases | R8 |
| S-phrases | S38 |
| Related compounds | |
| Related compounds | Nitric oxide, nitrogen dioxide, dinitrogen trioxide, dinitrogen tetroxide, dinitrogen pentoxide, nitric acid, nitrous acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Nitrous oxide, commonly known as "laughing gas", is a chemical compound with the chemical formula N2O. At room temperature, it is a colorless non-flammable gas, with a pleasant, slightly sweet odor and taste. It is used in surgery and dentistry for its anesthetic and analgesic effects. It is known as "laughing gas" due to the euphoric effects of inhaling it, a property that has led to its recreational use as an inhalant drug. It is also used in motor racing as an oxidizer to increase the power output of engines.
Contents |
Occurrence

Nitrous oxide is emitted by bacteria in soils and oceans, and thus has been a part of Earth's atmosphere for eons. Agriculture is the main source of human-produced nitrous oxide: cultivating soil, the use of nitrogen fertilizers, and animal waste handling can all stimulate naturally occurring bacteria to produce more nitrous oxide. The livestock sector (primarily cows, chickens, and pigs) produces 65% of human-related nitrous oxide. [1] Industrial sources make up only about 20% of all anthropogenic sources, and include the production of nylon, and the burning of fossil fuel in internal combustion engines. Human activity is thought to account for 30%; tropical soils and oceanic release account for 70%.[2]
Nitrous oxide reacts with ozone in the stratosphere. Nitrous oxide is the main naturally occurring regulator of stratospheric ozone. Nitrous oxide is a major greenhouse gas. Considered over a 100 year period, it has 298 times more impact per unit weight than carbon dioxide. Thus, despite its low concentration, nitrous oxide is the fourth largest contributor to these greenhouse gases. It ranks behind carbon dioxide, methane, and water vapor, the latter of which comprises greater than 95% of all greenhouse gases. Control of nitrous oxide is part of efforts to curb greenhouse gas emissions.
Manufacture
Nitrous oxide is most commonly prepared by careful heating of ammonium nitrate, which decomposes into nitrous oxide and water vapor.[3] The addition of various phosphates favors formation of a purer gas at slightly lower temperatures. One of the earliest commercial producers was George Poe in Trenton, New Jersey.[4]
- NH4NO3(s) → 2 H2O(g) + N2O(g)
This reaction occurs between 170 - 240°C, temperatures where ammonium nitrate is a moderately sensitive explosive and a very powerful oxidizer. Above 240 °C the exothermic reaction may accelerate to the point of detonation, so the mixture must be cooled to avoid such a disaster. Superheated steam is used to reach reaction temperature in some turnkey production plants.[5]
Downstream, the hot, corrosive mixture of gases must be cooled to condense the steam, and filtered to remove higher oxides of nitrogen. Ammonium nitrate smoke, as an extremely persistent colloid, will also have to be removed. The cleanup is often done in a train of 3 gas washes; namely base, acid and base again. Any significant amounts of nitric oxide (NO) may not necessarily be absorbed directly by the base (sodium hydroxide) washes.
The nitric oxide impurity is sometimes chelated out with ferrous sulfate, reduced with iron metal, or oxidised and absorbed in base as a higher oxide. The first base wash may (or may not) react out much of the ammonium nitrate smoke, however this reaction generates ammonia gas, which may have to be absorbed in the acid wash.
Other routes
The direct oxidation of ammonia may someday rival the ammonium nitrate pyrolysis synthesis of nitrous oxide mentioned above. This capital-intensive process, which originates in Japan, uses a manganese dioxide-bismuth oxide catalyst:[6]
- 2 NH3 + 2 O2 → N2O + 3 H2O
Higher oxides of nitrogen are formed as impurities. In comparison, uncatalyzed ammonia oxidation (i.e. combustion or explosion) goes primarily to N2 and H2O.
Nitrous oxide can be made by heating a solution of sulfamic acid and nitric acid. Many gases are made this way in Bulgaria.[7]
- HNO3 + NH2SO3H → N2O + H2SO4 + H2O
There is no explosive hazard in this reaction if the mixing rate is controlled. However, as usual, toxic higher oxides of nitrogen form.
Nitrous oxide is produced in large volumes as a by-product in the synthesis of adipic acid; one of the two reactants used in nylon manufacture.[8][9] This might become a major commercial source, but will require the removal of higher oxides of nitrogen and organic impurities. Currently much of the gas is decomposed before release for environmental protection. Greener processes may prevail that substitute hydrogen peroxide for nitric acid oxidation; hence no generation of oxide of nitrogen by-products.
Hydroxylammonium chloride can react with sodium nitrite to produce N2O as well:
- NH3OH+Cl− + NaNO2 → N2O + NaCl + H2O
If the nitrite is added to the hydroxylamine solution, the only remaining byproduct is salt water. However, if the hydroxylamine solution is added to the nitrite solution (nitrite is in excess), then toxic higher oxides of nitrogen are also formed.
Uses
Rocket motors
Nitrous oxide can be used as an oxidizer in a rocket motor. This has the advantages over other oxidizers that it is non-toxic and, due to its stability at room temperature, easy to store and relatively safe to carry on a flight. As a secondary benefit it can be readily decomposed to form breathing air. Its high density and low storage pressure enable it to be highly competitive with stored high-pressure gas systems.
In a 1914 patent, American rocket pioneer Robert Goddard suggested nitrous oxide and gasoline as possible propellants for a liquid-fueled rocket. Nitrous oxide has been the oxidizer of choice in several hybrid rocket designs (using solid fuel with a liquid or gaseous oxidizer). The combination of nitrous oxide with hydroxyl-terminated polybutadiene fuel has been used by SpaceShipOne and others. It is also notably used in amateur and high power rocketry with various plastics as the fuel. An episode of MythBusters featured a hybrid rocket built using a paraffin/powdered carbon mixture as its solid fuel and nitrous oxide as its oxidizer.
Nitrous oxide can also be used in a monopropellant rocket. In the presence of a heated catalyst, N2O will decompose exothermically into nitrogen and oxygen, at a temperature of approximately 1300 °C. Because of the large heat release the catalytic action rapidly becomes secondary as thermal autodecomposition becomes dominant. In a vacuum thruster, this can provide a monopropellant specific impulse (Isp) of as much as 180s. While noticeably less than the Isp available from hydrazine thrusters (monopropellant or bipropellant with nitrogen tetroxide), the decreased toxicity makes nitrous oxide an option worth investigating. Because of its release of very high temperature oxygen as a monopropellant the addition of even small amounts of a fuel such as hydrogen rapidly increases the specific impulse and the high oxygen temperatures simplify ignition of the fuel. Isp greater than 340 seconds can be readily achieved. Its low freezing point also eases thermal management as compared to hydrazine -- a valuable property on a spacecraft which may contain quantities of cryogenic propellant.
Internal combustion engine
In vehicle racing, nitrous oxide (often referred to as just "nitrous" in this context to differ from the acronym NOS which is the brand Nitrous Oxide Systems) is sometimes injected into the intake manifold (or prior to the intake manifold), whereas other systems directly inject right before the cylinder (direct port injection) to increase power. The gas itself is not flammable, but it delivers more oxygen than atmospheric air by breaking down at elevated temperatures, allowing the engine to burn more fuel and air and resulting in more powerful combustion. Nitrous oxide is stored as a compressed liquid; the evaporation and expansion of liquid nitrous oxide in the intake manifold causes a large drop in intake charge temperature, resulting in a denser charge, further allowing more air/fuel mixture to enter the cylinder.
The same technique was used during World War II by Luftwaffe aircraft with the GM 1 system to boost the power output of aircraft engines. Originally meant to provide the Luftwaffe standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialized planes like high-altitude reconnaissance aircraft, high-speed bombers and high-altitude interceptors.
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to damage or destroy the engine. Very large power increases are possible, and if the mechanical structure of the engine is not properly reinforced, the engine may be severely damaged or destroyed during this kind of operation. It is very important with nitrous oxide augmentation of internal combustion engines to maintain proper operating temperatures and fuel levels to prevent preignition, or detonation (sometimes referred to as knocking or pinging). Most problems that are associated with nitrous do not come from mechanical failure due to the power increases. Since nitrous allows a much denser charge into the cylinder it dramatically increases cylinder pressures. The increased pressure results in heat, and heat will cause many problems from melting the piston/Cylinder Head/valves, to predetonation.
Aerosol propellant

The gas is approved for use as a food additive (also known as E942), specifically as an aerosol spray propellant. Its most common uses in this context are in aerosol whipped cream canisters, cooking sprays, and as an inert gas used to displace bacteria-inducing oxygen when filling packages of potato chips and other similar snack foods.
The gas is extremely soluble in fatty compounds. In aerosol whipped cream, it is dissolved in the fatty cream until it leaves the can, when it becomes gaseous and thus creates foam. Used in this way, it produces whipped cream four times the volume of the liquid, whereas whipping air into cream only produces twice the volume. If air were used as a propellant, oxygen would accelerate rancidification of the butterfat; nitrous oxide inhibits such degradation. Carbon dioxide cannot be used for whipped cream because it is acidic in water, which would curdle the cream and give it a seltzer-like 'sparkling' sensation.
However, the whipped cream produced with nitrous oxide is unstable, and will return to a more or less liquid state within half an hour to one hour. Thus, the method is not suitable for decorating food that will not be immediately served. Similarly, cooking spray, which is made from various types of oils combined with lecithin (an emulsifier), may use nitrous oxide as a propellant; other propellants used in cooking spray include food-grade alcohol and propane.
Users of nitrous oxide often obtain it from whipped cream dispensers that use nitrous oxide as a propellant (see above section), for recreational use as a euphoria-inducing inhalant drug. It is non-harmful in small doses, but risks due to lack of oxygen do exist (see Recreational use below).
In medicine
Previously, nitrous oxide was typically administered by dentists through a demand-valve inhaler over the nose that only releases gas when the patient inhales through the nose; full-face masks are not commonly used by dentists, so that the patient's mouth can be worked on while the patient continues to inhale the gas. Current use involves constant supply flowmeters which allow the proportion of nitrous oxide and the combined gas flow rate to be individually adjusted. The masks still cover only the nose.
Because nitrous oxide is minimally metabolized, it retains its potency when exhaled into the room by the patient and can pose an intoxicating and prolonged-exposure hazard to the clinic staff if the room is poorly ventilated. Where nitrous oxide is administered, a continuous-flow fresh-air ventilation system or nitrous-scavenging system is used to prevent waste gas buildup.
Nitrous oxide is a weak general anesthetic, and so is generally not used alone in general anesthesia. In general anesthesia it is used as a carrier gas in a 2:1 ratio with oxygen for more powerful general anesthetic agents such as sevoflurane or desflurane. It has a MAC (minimum alveolar concentration) of 105% and a blood:gas partition coefficient of 0.46. Less than 0.004% is metabolised in humans.
Recreational use
Nitrous oxide (N2O) is a dissociative drug that can cause analgesia, depersonalization, derealization, dizziness, euphoria, and some sound distortion . [10]
Since the earliest uses of nitrous oxide for medical or dental purposes, it has also been used recreationally as an inhalant, because it causes euphoria and slight hallucinations. Only a small number of recreational users (such as dental office workers or medical gas technicians) have legal access to pure nitrous oxide canisters that are intended for medical or dental use. Most recreational users obtain nitrous oxide from compressed gas containers which use nitrous oxide as a propellant for whipped cream or from automotive nitrous systems. Automotive nitrous available to the public sometimes has ~100 ppm sulfur dioxide and/or elemental sulfur added to prevent recreational use/abuse; (not hydrogen sulfide as suggested by[11]). Inhalation of such a mixture is nearly impossible after one breath due to gagging and sooner or later, involuntary clamping off of the trachea; (some with "sulfite" allergies could even die due to allergic reaction).
Users typically inflate a balloon or a plastic bag with nitrous oxide from a tank or a one-use 'charger', and then inhale the gas for its effects. Nitrous oxide expelled directly from a tank or canister is extremely cold, and would severely damage the user's lungs. Recreational users typically do not mix it with air or oxygen and thus may risk injury, or death from anoxia.
Inhaling nitrous oxide in conjunction with an amyl nitrite (aka poppers) is in some circles referred to as "space surfing", as the nitrous oxide acts synergistically with the alkyl nitrite to create strong (but short-lived) euphoria, analgesia, dissociation, and in some cases, sensations of internal movement or agitation. The name also comes from the sound distorting effects of nitrous oxide, which some users compare to the sound of waves crashing on a beach (hence "surfing"). While powerful, this is a potentially dangerous combination, as the central nervous system (CNS) depressing effects of the nitrous oxide, combined with the drop in blood pressure (which is characteristic of nitrite inhalant use), may cause hypotension, unconsciousness, or, in the case of extreme overdose, death.
Nitrous oxide is used as a whipping agent due to the ease with which it migrates into and out of oils. Similarly, prolonged inhalation of high concentrations of nitrous oxide will cause it to migrate throughout the body into sinus cavities, the digestive tract, and into fat cells. An inactive person who has breathed high concentrations for 20-30 minutes but then breathes normally will still retain the gas in their body at low doses as the gas slowly migrates back out of these internal cavities. Even after several hours of not breathing the gas, sudden rapid whole-body movements such as calisthenics causes the dissolved gas to suddenly begin migrating out of fat cells, resulting in a latent dosing effect.
Nitrous oxide can be habit-forming because of its short-lived effect (generally from 0.1 - 1 minutes in recreational doses). Long-term use in excessive quantities has been associated with vitamin B12 deficiency anemia due to reduced hemopoiesis, neuropathy, tinnitus, and numbness in extremities. Pregnant women should not use nitrous oxide as chronic use is teratogenic and foetotoxic. One study in rats found that long term exposure to high doses of nitrous oxide may lead to Olney's lesions.[12]
Neuropharmacology

Nitrous oxide shares many pharmacological similarities with other inhaled anesthetics, but there are a number of differences. Nitrous oxide is relatively non-polar, has a low molecular weight, and high lipid solubility. As a result it can quickly diffuse into phospholipid cell membranes.
Like many classical anesthetics, the exact mechanism of action is still open to some conjecture. It antagonizes the NMDA receptor at partial pressures similar to those used in general anaesthesia. The evidence on the effect of N2O on GABA-A currently is mixed, but tends to show a lower potency potentiation.[13] N2O, like other volatile anesthetics, activates twin-pore potassium channels, albeit weakly. These channels are largely responsible for keeping neurons at the resting (unexcited) potential.[14] Unlike many anesthetics, however, N2O does not seem to affect calcium channels.[13]
Unlike most general anesthetics, N2O appears to affect the GABA receptor. In many behavioral tests of anxiety, a low dose of N2O is a successful anxiolytic. This anti-anxiety effect is partially reversed by benzodiazepine receptor antagonists. Mirroring this, animals which have developed tolerance to the anxiolytic effects of benzodiazepines are partially tolerant to nitrous oxide.[15] Indeed, in humans given 30% N2O, benzodiazepine receptor antagonists reduced the subjective reports of feeling “high”, but did not alter psycho-motor performance.[16]
The effects of N2O seem linked to the interaction between the endogenous opioid system and the descending noradrenergic system. When animals are given morphine chronically they develop tolerance to its analgesic (pain killing) effects; this also renders the animals tolerant to the analgesic effects of N2O.[17] Administration of antibodies which bind and block the activity of some endogenous opioids (not beta-endorphin), also block the antinociceptive effects of N2O.[18] Drugs which inhibit the breakdown of endogenous opioids also potentiate the antinociceptive effects of N2O.[18] Several experiments have shown that opioid receptor antagonists applied directly to the brain block the antinociceptive effects of N2O, but these drugs have no effect when injected into the spinal cord.
Conversely, alpha-adrenoreceptor antagonists block the antinociceptive effects of N2O when given directly to the spinal cord, but not when applied directly to the brain.[19] Indeed, alpha2B-adrenoreceptor knockout mice or animals depleted in noradrenaline are nearly completely resistant to the antinociceptive effects of N2O.[20] It seems N2O-induced release of endogenous opioids causes disinhibition of brain stem noradrenergic neurons, which release norepinephrine into the spinal cord and inhibit pain signaling (Maze, M. and M. Fujinaga, 2000). Exactly how N2O causes the release of opioids is still uncertain.
Safety
The major safety hazards of nitrous oxide come from the fact that it is a compressed liquefied gas, an asphyxiation risk, and a dissociative anaesthetic. Exposure to nitrous oxide causes short-term decreases in mental performance, audiovisual ability, and manual dexterity.[21]
A study of workers[22] and several experimental animal studies[23][24][25][26] indicate that adverse reproductive effects may also result from chronic exposure to nitrous oxide.
The National Institute for Occupational Safety and Health recommends that workers' exposure to nitrous oxide should be controlled during the administration of anesthetic gas in medical, dental, and veterinary operatories.[27]
Chemical/physical
At room temperature (20°C) the saturated vapour pressure is 58.5 bar, rising up to 72.45 bar at 36.4°C — the critical temperature. The pressure curve is thus unusually sensitive to temperature.[28] Liquid nitrous oxide acts as a good solvent for many organic compounds; liquid mixtures may form shock sensitive explosives.
As with many strong oxidisers, contamination of parts with fuels have been implicated in rocketry accidents, where small quantities of nitrous / fuel mixtures explode due to 'water hammer' like effects (sometimes called 'dieseling' — heating due to adiabatic compression of gases can reach decomposition temperatures).[29] Some common building materials such as stainless steel and aluminum can act as fuels with strong oxidisers such as nitrous oxide, as can contaminants, which can ignite due to adiabatic compression.[30]
There have also been accidents where nitrous oxide decomposition in plumbing has led to the explosion of large tanks.[31]
Biological
Nitrous oxide inactivates the cobalamin form of vitamin B12 by oxidation. Symptoms of vitamin B12 deficiency, including sensory neuropathy, myelopathy, and encephalopathy, can occur within days or weeks of exposure to nitrous oxide anesthesia in people with subclinical vitamin B12 deficiency. Symptoms are treated with high doses of vitamin B12, but recovery can be slow and incomplete[32] People with normal vitamin B12 levels have stores to make the effects of nitrous oxide insignificant, unless exposure is repeated and prolonged (nitrous oxide abuse). Vitamin B12 levels should be checked in people with risk factors for vitamin B12 deficiency prior to using nitrous oxide anesthesia.
Nitrous oxide has also been shown to induce early stages of Olney's lesions in the brains of rats. However none of the lesions found were irreversible.[12]
Legality
In the United States, possession of nitrous oxide is legal under federal law and is not subject to DEA purview.[33] It is, however, regulated by the Food and Drug Administration under the Food Drug and Cosmetics Act; prosecution is possible under its "misbranding" clauses, prohibiting the sale or distribution of nitrous oxide for the purpose of human consumption.
Many states have laws regulating the possession, sale, and distribution of nitrous oxide. Such laws usually ban distribution to minors or limit the amount of nitrous oxide that may be sold without special license. In most jurisdictions, such as at the federal level, sale or distribution for the purpose of recreational consumption is illegal.[33]
In some countries, it is illegal to have nitrous oxide systems plumbed into an engine's intake manifold. These laws are ostensibly used to prevent street racing and meet emission standards.
Nitrous oxide is entirely legal to possess and inhale in the United Kingdom.
In New Zealand, the Ministry of Health has warned that nitrous oxide is a prescription medicine, and its sale or possession without a prescription is an offense under the Medicines Act.[34] This statement would seemingly prohibit all non-medicinal uses of the chemical, though it is implied that only recreational use will be legally targeted.
History
The gas was first synthesized by English chemist and natural philosopher Joseph Priestley in 1775 [2], who called it phlogisticated nitrous air (see phlogiston). Priestley describes the preparation of "nitrous air diminished" by heating iron filings dampened with nitric acid in Experiments and Observations on Different Kinds of Air (1775). Priestley was delighted with his discovery: "I have now discovered an air five or six times as good as common air... nothing I ever did has surprised me more, or is more satisfactory."[35] Humphry Davy in the 1790s tested the gas on himself and some of his friends, including the poets Samuel Taylor Coleridge and Robert Southey.
They realized that nitrous oxide considerably dulled the sensation of pain, even if the inhaler were still semi-conscious. After it was publicized extensively by Gardner Quincy Colton in the United States in the 1840s, it came into use as an anaesthetic, particularly by dentists, who do not typically have access to the services of an anesthesiologist and who may benefit from a patient who can respond to verbal commands.
See Also
- Whipped-cream charger
References
- ↑ H. Steinfeld, P. Gerber, T. Wassenaar, V. Castel, M. Rosales, C. de Haan (2006). "Livestock’s long shadow -- Environmental issues and options". Retrieved on 2008-02-02.
- ↑ "Sources and Emissions -- Where Does Nitrous Oxide Come From?". U. S. Environmental Protection Agency (2006). Retrieved on 2008-02-02.
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ↑ "George Poe is Dead", Washington Post (February 3, 1914). Retrieved on 2007-12-29. "Cousin of Famous Poet and Noted as a Scientist. Inventor of the Respirator. Also First to Liquefy Nitrous Oxide. Cadet at Virginia Military Institute at Time of Battle of Newmarket. Mentioned for the Nobel Prize for Scientific Attainment in Chemistry. Prof. George Poe, a cousin of the poet Edgar Allan Poe, a noted scientist and inventor, who had been mentioned for the Nobel prize for scientific attainment, a former resident of Washington, died in Norfolk, Virginia, yesterday of general paralysis. Prof. Poe was in his sixty-eighth year."
- ↑ "Nitrous oxide plant". Sanghi Organization.
- ↑ Synthesis of Nitrous Oxide by Oxidation of Ammonia T Suwa, A Matsushima, Y Suziki, Y Namina - Kohyo Kagaku Zasshi, 1961; Showa Denka Ltd.
- ↑ Brozadzhiew & Rettos, 1975.
- ↑ Reimer R. A.; Slaten C. S.; Seapan M.; Lower M. W.; Tomlinson P. E.; (1994). "Abatement of N2O emissions produced in the adipic acid industry". Environmental progress 13 (2): 134–137. doi:.
- ↑ .A. Shimizu, , K. Tanaka and M. Fujimori (2000). "Abatement of N2O emissions produced in the adipic acid industry". Chemosphere - Global Change Science 2 (3-4): 425–434. doi:.
- ↑ AJ Giannini. Volatiles. In NS Miller (Ed.). A Comprehensive Handbook of Drug and Alcohol Addiction. NY, Marcel Dekker, 1991.
- ↑ "Obtaining Nitrous Oxide". Just Say N2O. Retrieved on 2008-02-02.
- ↑ 12.0 12.1 Jevtovic-Todorovic V, Beals J, Benshoff N, Olney J (2003). "Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain". Neuroscience 122 (3): 609–16. doi:. PMID 14622904.
- ↑ 13.0 13.1 Mennerick, S., Jevtovic-Todorovic, V., Todorovic, S.M., Shen, W., Olney, J.W. & Zorumski, C.F. (1998). "Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures". Journal of Neuroscience 18 (23): 9716–26. PMID 9822732. http://www.jneurosci.org/cgi/content/abstract/18/23/9716.
- ↑ Gruss, M., Bushell, T.J., Bright, D.P., Lieb, W.R., Mathie, A. & Franks, N.P. (2004). "Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane". Molecular Pharmacology 65: 443–52.
- ↑ Emmanouil, D.E., Johnson, C.H. & Quock, R.M. (1994). "Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors". Psychopharmacology 115 (1-2): 167–72. doi:.
- ↑ Zacny, J.P., Yajnik, S., Coalson, D., Lichtor, J.L., Apfelbaum, J.L., Rupani, G., Young, C., Thapar, P. & Klafta, J. (1995). "Flumazenil may attenuate some subjective effects of nitrous oxide in humans: a preliminary report". Pharmacology Biochemistry and Behavior 51 (4): 815–9. doi:. PMID 7675863.
- ↑ Berkowitz, B.A., Finck, A.D., Hynes, M.D. & Ngai, S.H. (1979). "Tolerance to nitrous oxide analgesia in rats and mice". Anesthesiology (51): 309–12. doi:.
- ↑ 18.0 18.1 Branda, E.M., Ramza, J.T., Cahill, F.J., Tseng, L.F. & Quock, R.M. (2000). "Role of brain dynorphin in nitrous oxide antinociception in mice". Pharmacology Biochemistry and Behavior 65: 217–21. doi:.
- ↑ Guo, T.Z., Davies, M.F., Kingery, W.S., Patterson, A.J., Limbird, L.E. & Maze, M. (1999). "Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice". Anesthesiology 90: 470–6. doi:. PMID 9952154. http://www.anesthesiology.org/pt/re/anes/abstract.00000542-199902000-00022.htm.
- ↑ Sawamura, S., Kingery, W.S., Davies, M.F., Agashe, G.S., Clark, J.D., Koblika, B.K., Hashimoto, T. & Maze, M. (2000). "Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha2B adrenoceptors]". J. Neurosci. 20 (24): 9242–51. PMID 11125002. http://www.jneurosci.org/cgi/content/abstract/20/24/9242.
- ↑ Criteria for a recommended standard: occupational exposure to waste anesthetic gases and vapors. Cincinnati, OH: U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, DHEW (NIOSH) Publication No. 77B140.
- ↑ Rowland AS, Baird DD, Weinberg CR, Shore DL, Shy CM, Wilcox AJ [1992]. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. New Eng J Med 327(14):993B997.
- ↑ Corbett TH, Cornell RG, Endres JL, Millard RI [1973]. Effects of low concentrations of nitrous oxide on rat pregnancy. Anesthesiology 39:299B301.
- ↑ Vieira E [1979]. Effect of the chronic administration of nitrous oxide 0.5% to gravid rats. Br J Anaesth 51:283B287.
- ↑ Vieira E, Cleaton-Jones JP, Austin JC, Moyes DG, Shaw R [1980]. Effects of low concentrations of nitrous oxide on rat fetuses. Anesth and Analgesia 59(3):175B177.
- ↑ Vieira E, Cleaton-Jones P, Moyes D [1983]. Effects of low intermittent concentrations of nitrous oxide on the developing rat fetus. Br J Anaesth 55:67B69.
- ↑ NIOSH Alert: Controlling Exposures to Nitrous Oxide During Anesthetic Administration. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 94-100 [1]
- ↑ Air Liquid data on Nitrous oxide
- ↑ vaseline triggered explosion of hybrid rocket
- ↑ Safetygram 20: Nitrous Oxide
- ↑ Nitrous Oxide Trailer Rupture July 2, 2001 Report at CGA Seminar “Safety and Reliability of Industrial Gases, Equipment and Facilities”, October 15 -17, 2001, St. Louis, Missouri by Konrad Munke, LindeGas AG
- ↑ AJ Giannini. Drug Abuse. Los Angeles, Health Information Press,1999.
- ↑ 33.0 33.1 Center for Cognitive Liberty and Ethics: State Laws Concerning Inhalation of Nitrous Oxide
- ↑ Beehive.govt.nz - Time's up for sham sales of laughing gas
- ↑ J. R. Partington, A Short History of Chemistry, 3rd ed., Dover Publications, Inc., New York, New York, 1989, pp. 110-121.
External links
- Occupational Safety and Health Guideline for Nitrous Oxide
- Paul Crutzen Interview Freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust.
- National Pollutant Inventory - Oxide of nitrogen fact sheet
- National Institute for Occupational Safety and Health - Nitrous Oxide
- Erowid article on Nitrous Oxide
|
|||||||||||
|
|||||
|
|||||||||||||||||||||||||||