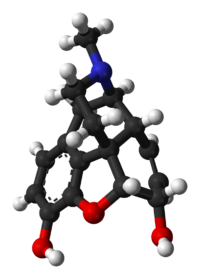
Morphine
 |
|
 |
|
|
Morphine
|
|
| Systematic (IUPAC) name | |
| (5α,6α)-7,8-didehydro- 4,5-epoxy-17-methylmorphinan-3,6-diol |
|
| Identifiers | |
| CAS number | |
| ATC code | N02 |
| PubChem | |
| DrugBank | |
| ChemSpider | |
| Chemical data | |
| Formula | C17H19NO3 |
| Mol. mass | 285.4 |
| Pharmacokinetic data | |
| Bioavailability | ~25% (oral); 100% (IV); |
| Protein binding | 30–40% |
| Metabolism | Hepatic 90% |
| Half life | 2–3 hours |
| Excretion | Renal 90%, biliary 10% |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Controlled (S8)(AU) Schedule I(CA) Class A(UK) Schedule II(US) |
| Dependence Liability | Extremely high |
| Routes | Smoked/inhaled, insufflated, Oral, SC, IM, IV |
Indicated for:
Recreational uses:
Other uses:
|
Contraindications, relative:
|
| Side effects:
Severe:
Atypical sensations:
Eye:
Hepatological:
Respiratory:
Skin:
|
Morphine (INN) (pronounced /ˈmɔrfiːn/) is a highly potent opiate analgesic drug and is the principal active agent in opium and the prototypical opioid. It is also a natural endocrine product in humans and other animals. Like other opioids, e.g. diacetylmorphine (heroin), morphine acts directly on the central nervous system (CNS) to relieve pain, and at synapses of the nucleus accumbens in particular. Morphine is highly addictive when compared to other substances; tolerance, physical and psychological dependence develops very rapidly.
Contents |
Medical uses
Morphine can be used:
- as an analgesic in hospital settings to relieve
- pain in myocardial infarction
- pain in sickle cell crisis
- pain associated with surgical conditions, pre- and postoperatively
- pain associated with trauma
- in the relief of severe chronic pain, e.g.,
- cancer
- pain from kidney stones (renal colic, ureterolithiasis)
- severe back pain
- as an adjunct to general anesthesia
- in epidural anesthesia or intrathecal analgesia
- for palliative care (i.e., to alleviate pain without curing the underlying reason for it, usually because the latter is found impossible)
- as an antitussive for severe cough
- in nebulized form, for treatment of dyspnea, although the evidence for efficacy is slim.[1] Evidence is better for other routes.[2]
- as an antidiarrheal in chronic conditions (e.g., for diarrhea associated with AIDS, although loperamide (a non-absorbed opioid acting only on the gut) is the most commonly used opioid for diarrhea).
Contraindications
The following conditions are relative contraindications for morphine:
- acute respiratory depression
- renal failure (due to accumulation of the metabolite morphine-6-glucuronide)
- chemical toxicity (potentially lethal in low tolerance subjects)
- raised intracranial pressure, including head injury (exacerbation due pCO2 increases from respiratory depression)
Older literature, based upon studies of animals with acute pancreatitis, claimed that morphine caused significant spasm of the sphincter of Oddi and could therefore worsen the pain of the disease.
Further information
See Opioids
Pharmacology
Morphine is the prototype narcotic drug and is the standard against which all other opioids are tested. It interacts predominantly with the μ-opioid receptor. These μ-binding sites are discretely distributed in the human brain, with high densities in the posterior amygdala, hypothalamus, thalamus, nucleus caudatus, putamen, and certain cortical areas. They are also found on the terminal axons of primary afferents within laminae I and II (substantia gelatinosa) of the spinal cord and in the spinal nucleus of the trigeminal nerve.[3]
Morphine is a phenanthrene opioid receptor agonist – its main effect is binding to and activating the μ-opioid receptors in the central nervous system. In clinical settings, morphine exerts its principal pharmacological effect on the central nervous system and gastrointestinal tract. Its primary actions of therapeutic value are analgesia and sedation. Activation of the μ-opioid receptors is associated with analgesia, sedation, euphoria, physical dependence, and respiratory depression. Morphine is a rapid-acting narcotic, and it is known to bind very strongly to the μ-opioid receptors, and for this reason, it often has a higher incidence of euphoria/dysphoria, respiratory depression, sedation, pruritus, tolerance, and physical and psychological dependence when compared to other opioids at equianalgesic doses. Morphine is also a κ-opioid and δ-opioid receptor agonist, κ-opioid's action is associated with spinal analgesia, miosis (pinpoint pupils) and psychotomimetic effects. δ-opioid is thought to play a role in analgesia.[4]
The effects of morphine can be countered with opioid antagonists such as naloxone and naltrexone; the development of tolerance to morphine may be inhibited by NMDA antagonists such as ketamine or dextromethorphan.[5]
Morphine is primarily metabolized into morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G)[6] via glucuronidation by phase II metabolism enzyme UDP-glucuronosyl transferase-2B7 (UGT2B7). The cytochrome P450 (CYP) family of enzymes involved in phase I metabolism plays a lesser role. Not only does the metabolism occur in the liver but it may also take place in the brain and the kidneys. M6G has been found to be a far more potent analgesic than morphine when dosed to rodents, but crosses the blood-brain barrier with difficulty. M6G has been shown to be relatively more selective for mu-receptors than for delta- and kappa-receptors, whereas M3G does not appear to compete for opioid receptor binding. The significance of M6G formation on the observed effect of a dose of morphine is the subject of extensive debate among pharmacologists.
Constipation
Like loperamide and other opioids, morphine acts on the myenteric plexus in the intestinal tract, reducing gut motility, causing constipation. The gastrointestinal effects of morphine are mediated primarily by μ-opioid receptors in the bowel. By inhibiting gastric emptying and reducing propulsive peristalsis of the intestine, morphine decreases the rate of intestinal transit. Reduction in gut secretion and increases in intestinal fluid absorption also contribute to the constipating effect. Opioids also may act on the gut indirectly through tonic gut spasms after inhibition of nitric oxide generation. This effect was shown in animals when a nitric oxide precursor reversed morphine-induced changes in gut motility.
Gene expression
Studies have shown that morphine can alter the expression of a number of genes. A single injection of morphine has been shown to alter the expression of two major groups of genes, for proteins involved in mitochondrial respiration and for cytoskeleton-related proteins.[7]
Effects on the immune system
Morphine has long been known to act on receptors expressed on cells of the central nervous system resulting in pain relief and analgesia. In the 1970s and '80s, evidence suggesting that opiate drug addicts show increased risk of infection (such as increased pneumonia, tuberculosis, and HIV) led scientists to believe that morphine may also affect the immune system. This possibility increased interest in the effect of chronic morphine use on the immune system.
The first step of determining that morphine may affect the immune system was to establish that the opiate receptors known to be expressed on cells of the central nervous system are also expressed on cells of the immune system. One study successfully showed that dendritic cells, part of the innate immune system, display opiate receptors. Dendritic cells are responsible for producing cytokines, which are the tools for communication in the immune system. This same study showed that dendritic cells chronically treated with morphine during their differentiation produce more interleukin-12 (IL-12), a cytokine responsible for promoting the proliferation, growth, and differentiation of T-cells (another cell of the adaptive immune system) and less interleukin-10 (IL-10), a cytokine responsible for promoting a B-cell immune response (B cells produce antibodies to fight off infection).[8]
This regulation of cytokines appear to occur via the p38 MAPKs (mitogen activated protein kinase) dependent pathway. Usually, the p38 within the dendritic cell expresses TLR 4 (toll-like receptor 4), which is activated through the ligand LPS (lipopolysaccharide). This causes the p38 MAPK to be phosphorylated. This phosphorylation activates the p38 MAPK to begin producing IL-10 and IL-12. When the dendritic cell is chronically exposed to morphine during their differentiation process then treated with LPS, the production of cytokines is different. Once treated with morphine, the p38 MAPK does not produce IL-10, instead favoring production of IL-12. The exact mechanism through which the production of one cytokine is increased in favor over another is not known. Most likely, the morphine causes increased phosphorylation of the p38 MAPK. Transcriptional level interactions between IL-10 and IL-12 may further increase the production of IL-12 once IL-10 is not being produced. Future research may target the exact mechanism that increases the production of IL-12 in morphine treated dendritic cells. This increased production of IL-12 causes increased T-cell immune response. This response is due to the ability of IL-12 to cause T helper cells to differentiate into the Th1 cell, causing a T cell immune response.
Chemistry

Morphine is a benzylisoquinoline alkaloid with two additional ring closures.
Most of the licit morphine produced is used to make codeine by methylation. It is also a precursor for heroin (diacetylmorphine), hydromorphone, and oxymorphone. Replacement of the N-methyl group of morphine with an N-phenylethyl group results in a product that is 18 times more powerful than morphine in its opiate agonist potency. Combining this modification with the replacement of the 6-hydroxyl with a 6-methylene produces a compound some 1,443 times more potent than morphine, stronger than the Bentley compounds such as etorphine.
Both morphine and its hydrated form, C17H19NO3H2O, are sparingly soluble in water. In five liters of water, only one gram of the hydrate will dissolve. For this reason, pharmaceutical companies produce sulfate and hydrochloride salts of the drug, both of which are over 300 times more water-soluble than their parent molecule. Whereas the pH of a saturated morphine hydrate solution is 8.5, the salts are acidic. Since they derive from a strong acid but weak base, they are both at about pH = 5; as a consequence, the morphine salts are mixed with small amounts of NaOH to make them suitable for injection.[9]
A number of salts of morphine are used, and the opioids Morphine-N-Oxide (Genomorphine) and Pseudomorphine form as degradation products of morphine. The salts listed by the United States Drug Enforcement Administration, in addition to a few others, are as follows:
| Salt or drug | CSA schedule | ACSCN | Free base conversion ratio |
|---|---|---|---|
| Morphine | II | 9300 | 1 |
| Morphine acetate | II | 9300 | 0.71 |
| Morphine citrate | II | 9300 | 0.81 |
| Morphine bitartrate | II | 9300 | 0.66 |
| Morphine stearate | II | 9300 | 0.51 |
| Morphine phthalate | II | 9300 | 0.89 |
| Morphine hydrobromide | II | 9300 | 0.78 |
| Morphine hydrobromide (2 H2O) | II | 9300 | 0.71 |
| Morphine hydrochloride | II | 9300 | 0.89 |
| Morphine hydrochloride (3 H2O) | II | 9300 | 0.76 |
| Morphine hydriodide (2 H2O) | II | 9300 | 0.64 |
| Morphine lactate | II | 9300 | 0.76 |
| Morphine monohydrate | II | 9300 | 0.94 |
| Morphine meconate (5 H2O) | II | 9300 | 0.66 |
| Morphine mucate | II | 9300 | 0.57 |
| Morphine nitrate | II | 9300 | 0.82 |
| Morphine phosphate (1/2 H2O) | II | 9300 | 0.73 |
| Morphine phosphate (7 H2O) | II | 9300 | 0.73 |
| Morphine salicylate | II | 9300 | |
| Morphine phenylpropionate | II | 9300 | 0.65 |
| Morphine methyliodide | II | 9300 | 0.67 |
| Morphine isobutyrate | II | 9300 | 0.76 |
| Morphine hypophosphite | II | 9300 | 0.81 |
| Morphine sulfate (5 H2O) | II | 9300 | 0.75 |
| Morphine tannate | II | 9300 | |
| Morphine tartrate (3 H2O) | II | 9300 | 0.74 |
| Morphine valerate | II | 9300 | 0.74 |
| Morphine methylbromide | I | 9305 | 0.75 |
| Morphine methylsulfonate | I | 9306 | 0.75 |
| Morphine-N-oxide | I | 9307 | 1 |
| Morphine-N-oxide quinate | I | 9307 | 0.60 |
| Pseudomorphine | I | not mentioned |
Production
A Hungarian chemist, János Kabay, found and internationally patented a method to extract morphine from "poppy straw": dried poppy pods and stem, and other parts of the dry plant, except for seeds and root. In natural form, in poppy plant, the alkaloids are bound to meconic acid. The method is to extract from the crushed plant with diluted sulfuric acid, which is a stronger acid than meconic acid, but not so strong to react with alkaloid molecules. The extraction is performed in many steps (one amount of crushed plant is at least six to ten times extracted, so practically every alkaloid goes into the solution). From the solution obtained at the last extraction step, the alkaloids are precipitated by either ammonium hydroxide or sodium carbonate. The last step is purifying and separating morphine from other opium alkaloids (opium poppy contains at least 15–20 different alkaloids, but most of them are of very low concentration). In the 1950s and 1960s, Hungary supplied nearly 60% of Europe's total medication-purpose morphine production. To this day, poppy farming is legal in Hungary, but poppy farms are limited by law to 2 acres (8,100 m2). It is also legal to sell dried poppy in flower shops for use in floral arrangements.
It was announced in 1973 that a team at the National Institutes of Health in the United States had developed a method for total synthesis of morphine, using coal tar as a starting material. A shortage in codeine-hydrocodone class cough suppressants (all of which can be made from morphine in one or more steps) was the initial reason for the research.
Legal classification
- In the United Kingdom, morphine is listed as a Class A drug under the Misuse of Drugs Act 1971 and a Schedule 2 Controlled Drug under The Misuse of Drugs Regulations 2001.
- In the United States, morphine is classified as a Schedule II drug under the Controlled Substances Act.
- In Canada, morphine is classified as a Schedule I drug under the Controlled Drugs and Substances Act.
- In Australia, morphine is classified as a Schedule 8 drug under the variously titled State and Territory Poisons Acts.
- In the Netherlands, morphine is classified as a List 1 drug under the Opium Law.
- Internationally, morphine is a Schedule I drug under the Single Convention on Narcotic Drugs.[10]
History and non-medical use

Morphine was first isolated in 1804 in Paderborn, Germany,[12] by the German pharmacist Friedrich Wilhelm Adam Sertürner, who named it morphium after Morpheus, the Greek god of dreams. But it was not until the development of the hypodermic needle in 1853 that its use spread.[13] It was used for pain relief, and as a "cure" for opium and alcohol addiction. Later it was found out that morphine was even more addictive than either alcohol or opium, and its extensive use during the American Civil War allegedly resulted in over 400,000[14] sufferers from the "soldier's disease" of morphine addiction.[15] [16] This idea has been a subject of controversy, as there have been suggestions that such a disease was in fact a hoax.[17][18]
Diacetylmorphine (better known as heroin) was synthesized from morphine in 1874 and brought to market by Bayer in 1898. Heroin is approximately 1.5–2 times more potent than morphine on a milligram-for-milligram basis. Using a variety of subjective and objective measures, one study estimated the relative potency of heroin to morphine administered intravenously to post-addicts to be 1.80–2.66 mg of morphine sulfate to 1 mg of diamorphine hydrochloride (heroin).[19] The pharmacology of heroin and morphine is identical except the two acetyl groups increase the lipid solubility of the heroin molecule, causing it to cross the blood-brain barrier and enter the brain more rapidly. Once in the brain, these acetyl groups are removed to yield morphine, which causes the subjective effects of heroin. Thus, heroin may be thought of as a more rapidly acting form of morphine.[20] Morphine was the most commonly abused narcotic analgesic in the world up until heroin was synthesized and came into use. Even today, morphine is the most sought after prescription narcotic by heroin addicts when heroin is scarce.
Morphine, heroin and cocaine became controlled substances in the U.S. under the Harrison Narcotics Tax Act of 1914, and possession without a prescription in the U.S. is a criminal offense.
In 1952, Dr. Marshall D. Gates, Jr. was the first person to chemically synthesize morphine at the University of Rochester. This breakthrough is well renowned in the field of organic chemistry.[21]
Morphine is routinely carried by soldiers on operations in an autoinjector.
Slang terms for morphine include M, Big M, Vitamin M, Miss Emma, morph, morpho, Murphy, cube, cube juice, White Nurse, Red Cross, mojo, hocus, 13, Number 13, mofo, unkie, happy powder, joy powder, first line, Aunt Emma, coby, em, emsel, morf, dope, glad stuff, goody, God's Medicine, God's Own Medicine, hard stuff, morfa, morphia, morphy, mud, sister, Sister Morphine, stuff, white stuff, white merchandise and others.
Addiction

Morphine is a highly addictive substance, both psychologically and physically, with an addiction potential identical to that of heroin. In controlled studies comparing the physiological and subjective effects of injected heroin and morphine in individuals formerly addicted to opiates, subjects showed no preference for either drug. Equipotent, injected doses had comparable action courses, with no difference in their ability to induce euphoria, ambition, nervousness, relaxation, drowsiness, or sleepiness.[22] Data acquired from short-term addiction studies did not indicate that heroin tolerance develops more rapidly than tolerance to morphine. The findings have been discussed in relation to the physicochemical properties of heroin and morphine and the metabolism of heroin. When compared to other opioids — hydromorphone, fentanyl, oxycodone, and pethidine/meperidine, former addicts showed a strong preference for heroin and morphine, suggesting that heroin and morphine are particularly susceptible to abuse and addiction. Morphine and heroin were also much more likely to produce euphoria, and other subjective effects when compared to most opioid analgesics.[23][24]
Other studies such as the Rat Park experiments suggest that morphine is less physically addictive than others suggest, and most studies on morphine addiction merely show that "severely distressed animals, like severely distressed people, will relieve their distress pharmacologically if they can." [25] In these studies rats with a morphine "addiction" overcome their addiction themselves when placed in decent living environments with enough space, good food, companionship, areas for exercise, areas for privacy. More recent research has shown that an enriched environment may decrease morphine addiction in mice [2].
Withdrawal syndrome
The withdrawal symptoms associated with morphine addiction are usually experienced shortly before the time of the next scheduled dose, sometimes within as early as a few hours (usually between 6–12 hours) after the last administration. Early symptoms include watery eyes, insomnia, diarrhea, runny nose, yawning, dysphoria, and sweating and in some cases a strong drug craving. Severe headache, restlessness, irritability, loss of appetite, body aches, severe abdominal pain, nausea and vomiting, tremors, and even stronger and more intense drug craving appear as the syndrome progresses. Severe depression and vomiting are very common. The heart rate and blood pressure are elevated and can lead to a heart attack, blood clot or stroke. Chills or cold flashes with goose bumps ("cold turkey") alternating with flushing (hot flashes), kicking movements of the legs ("kicking the habit"[26]) and excessive sweating are also characteristic symptoms.[27] Severe pains in the bones and muscles of the back and extremities occur, as do muscle spasms. At any point during this process, a suitable narcotic can be administered that will dramatically reverse the withdrawal symptoms. Major withdrawal symptoms peak between 48 and 96 hours after the last dose and subside after about 8 to 12 days. Sudden withdrawal by heavily dependent users who are in poor health is very rarely fatal. Morphine withdrawal is considered less dangerous than alcohol, barbiturate, or benzodiazepine withdrawal.[28]
The psychological dependence associated with morphine addiction is complex and protracted. Long after the physical need for morphine has passed, the addict will usually continue to think and talk about the use of morphine (or other drugs) and feel strange or overwhelmed coping with daily activities without being under the influence of morphine. Psychological withdrawal from morphine is a very long and painful process.[29] Addicts often suffer severe depression, anxiety, insomnia, mood swings, amnesia (forgetfulness), low self-esteem, confusion, paranoia, and other psychological disorders. The psychological dependence on morphine can, and usually does, last a lifetime.[30] There is a high probability that relapse will occur after morphine withdrawal when neither the physical environment nor the behavioral motivators that contributed to the abuse have been altered. Testimony to morphine's addictive and reinforcing nature is its relapse rate. Abusers of morphine (and heroin), have one of the highest relapse rates among all drug users.
Hepatitis C and morphine withdrawal
Researchers at the University of Pennsylvania have demonstrated that morphine withdrawal complicates hepatitis C by suppressing IFN-alpha-mediated immunity and enhancing virus replication. Hepatitis C virus (HCV) is common among intravenous drug users, with 70 to 80% of abusers infected in the United States. This high association has piqued interest in determining the effects of drug abuse, specifically morphine and heroin, on progression of the disease. The discovery of such an association would impact treatment of both HCV infection and drug abuse.[31]
Access to morphine in poor countries
Although morphine is cheap, people in poorer countries often do not have access to it. According to a 2005 estimate by the International Narcotics Control Board, six countries (Australia, Britain, Canada, France, Germany, and the United States) consume 79 percent of the world’s morphine. The less affluent countries, accounting for 80 percent of the world's population, consumed only about 6 percent of the global morphine supply. Some countries import virtually no morphine, and in others the drug is rarely available even for relieving severe pain while dying. Experts in pain management attribute the under-distribution of morphine to an unwarranted fear of the drug's potential for addiction and abuse. While morphine is clearly addictive, western doctors believe it is worthwhile to use the drug and then wean the patient off when the treatment is over.[32]
Additional images
See also
- Heroin
- Cheese (recreational drug)
- Diacetyldihydromorphine
- Dihydromorphine
- Dipropanoylmorphine
- Drug addiction
- Drug injection
- Drugs and prostitution
- Entomotoxicology
- Illegal drug trade
- Methadone
- Monoacetylmorphine
- Opioid
- Opium
- Opium licensing
- Opium poppy
- Polish heroin
- Psychoactive drug
- Recreational drug use
References
- ↑ Nebulised morphine for dyspnoea
- ↑ Clinical knowledge Summaries
- ↑ MS-Contin (Morphine) clinical pharmacology - prescription drugs and medications at RxList
- ↑ MS-Contin (Morphine) clinical pharmacology - prescription drugs and medications at RxList
- ↑ Herman BH, Vocci F, Bridge P. "The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction." Neuropsychopharmacology. 1995 Dec;13(4):269-93. PMID 8747752
- ↑ Kilpatrick G.J. and Smith T.W. (2005). "Morphine-6-glucuronide: actions and mechanisms". Med. Res. Rev. 25 (5): 521–544. doi:. PMID 15952175.
- ↑ Loguinov A, Anderson L, Crosby G, Yukhananov R (2001). "Gene expression following acute morphine administration". Physiol Genomics 6 (3): 169–81. PMID 11526201.
- ↑ Messmer D, Hatsukari I, Hitosugi N, Schmidt-Wolf IG, Singhal PC. "Morphine reciprocally regulates IL-10 and IL-12 production by monocyte-derived human dendritic cells and enhances T cell activation." Molecular Medicine. 2006 Nov-Dec;12(11-12):284-90. PMID 17380193.
- ↑ Morphine
- ↑ [1]
- ↑ . Overland Monthly XXXV (205): xiv. January 1900.
- ↑ Dem Morphin auf der Spur
- ↑ Who Invented the Hypodermic Needle or Syringe Needle
- ↑ ASA July 2004 Newsletter
- ↑ Canadian Government Commission - Opiate Narcotics
- ↑ Old Soldiers Disease
- ↑ Mythical Roots of US Drug Policy - Soldier's Disease and Addicts in the Civil War
- ↑ Soldiers Disease A Historical Hoax?
- ↑ Martin WR, Fraser HF. "A comparative study of physiological and subjective effects of heroin and morphine administered intravenously in postaddicts." Journal of Pharmacology and Experimental Therapeutics. 1961 Sep;133:388-99. PMID 13767429
- ↑ Klous MG, Van den Brink W, Van Ree JM, Beijnen JH. "Development of pharmaceutical heroin preparations for medical co-prescription to opioid dependent patients." Drug and Alcohol Dependence. 2005 December 12;80(3):283-95. PMID 15916865. doi:10.1016/j.drugalcdep.2005.04.008
- ↑ University of Rochester Press Releases
- ↑ W. R. Martin 1 and H. F. Fraser 1
- ↑ 1 National Institute of Mental Health, Addiction Research Center, U. S. Public Health Service Hospital, Lexington, Kentucky
- ↑ Journal of Pharmacology And Experimental Therapeutics, Vol. 133, Issue 3, pp. 388-399, 1961
- ↑ Weissman, D. E. & Haddox, J. D. (1989). "Opioid pseudoaddiction: an iatrogenic syndrome," Pain, 36, 363-366, cited in Alexander 2001, op cit.
- ↑ Heroin Information from the National Institute on Drug Abuse
- ↑ Drugs and Human Performance FACT SHEETS - Morphine (and Heroin)
- ↑ DEA Briefs & Background, Drugs and Drug Abuse, Drug Descriptions, Narcotics
- ↑ Morphine withdrawal and depression
- ↑ O'Neal, Maryadele J. Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Merck. October 18, 2006.
- ↑ Wang C-Q, Li Y, Douglas SD, Wang X, Metzger DS, Zhang T, Ho W-Z: Morphine withdrawal enhances hepatitis C virus (HCV) replicon expression. Am J Pathol 2005, 167:1333-1340
- ↑ Donald G. McNeil Jr. (2007-09-10). "Drugs Banned, Many of World’s Poor Suffer in Pain", New York Times. Retrieved on 2007-09-11.
|
|||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||