Monosodium glutamate
| Monosodium glutamate | |
|---|---|
 |
|
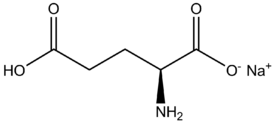
| IUPAC name | Sodium (2S)-2-amino-5-hydroxy-5-oxo-pentanoate |
| Identifiers | |
| CAS number | 142-47-2 |
| PubChem | |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C5H8NNaO4 |
| Molar mass | 169.111 |
| Appearance | white crystalline powder |
| Melting point |
225℃ |
| Solubility in water | very soluble in water |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|

Monosodium glutamate, also known as sodium glutamate and MSG, is a sodium salt of the non-essential amino acid glutamic acid. It is used as a food additive and is commonly marketed as a flavour enhancer. It has the HS code 29224220 and the E number E621. Trade names of monosodium glutamate include Ajinomoto, Vetsin, and Accent.
Although traditional Asian cuisine had often used seaweed extract, which contains high concentrations of glutamic acid, MSG was not isolated until 1907. MSG was subsequently patented by Ajinomoto Corporation of Japan in 1909. In its pure form, it appears as a white crystalline powder; when dissolved in water or saliva, it rapidly dissociates into sodium cations and glutamate anions (glutamate is the anionic form of glutamic acid, a naturally occurring amino acid).
Contents |
Production and chemical properties
MSG is prepared by the fermentation of carbohydrates, using bacteria species from genera such as Brevibacterium, Arthrobacter, Microbacterium, and Corynebacterium are useful. Yields of 100 g/litre can be prepared in this way. From 1909 to the mid 1960s, MSG was prepared by the hydrolysis of wheat gluten, which is roughly 25% glutamic acid. Glutamic acid is one of the least soluble amino acids, thus facilitating its purification.[1]
Like the sodium salts of other amino acids, MSG is a stable colourless solid that is degraded by strong oxidizing agents. It exists as enantiomers, but only the naturally occurring L-glutamate form is used as a flavour enhancer.
Commercialization
The Ajinomoto company was formed to manufacture and market MSG in Japan; the name 'Ajinomoto' means "essence of taste". It was introduced to the United States in 1947 as Ac'cent flavor enhancer.
Modern commercial MSG is produced by fermentation[2] of starch, sugar beets, sugar cane, or molasses. About 1.5 million metric tons were sold in 2001, with 4% annual growth expected.[3] MSG is used commercially as a flavour enhancer. Although once associated with foods in Chinese restaurants, MSG is now found frequently in many fast food chains and many common household food items, particularly processed foods. [4]
Examples include:
- Pre-prepared stocks often known as stock cubes or bouillon cubes.
- Condiments such as barbecue sauce and salad dressing.
- Canned, frozen, or dried prepared food
- Common snack foods such as flavored potato chips and flavored tortilla chips.
- Seasoning mixtures
Only the L-glutamate enantiomer has flavour-enhancing properties.[5] Manufactured MSG contains over 99.6% of the naturally predominant L-glutamate form, which is a higher proportion of L-glutamate than found in the free glutamate ions of naturally occurring foods. Fermented products like soy sauce, steak sauce, and Worcestershire sauce have comparable levels of glutamate as foods with added MSG. However, glutamate in these brewed products may be composed 5% or more of the D-enantiomer.[5]
Health controversy
Monosodium glutamate as a food ingredient is the subject of a health concern controversy.
United States
Monosodium glutamate is one of several forms of free glutamate used in foods. Free glutamate can also be present in a wide variety of other additives, including hydrolyzed vegetable proteins, autolyzed yeast, hydrolyzed yeast, yeast extract, soy extracts, and protein isolate, any one of which may appear as "spices" or "natural flavorings." The food additives disodium inosinate and disodium guanylate are usually used along with with monosodium glutamate-containing ingredients, and provide a likely indicator of the presence of monosodium glutamate in a product. For this reason, the FDA considers labels such as "No MSG" or "No Added MSG" to be misleading if the food contains ingredients that are sources of free glutamate, such as hydrolyzed protein.[6]
In 1993, FDA proposed adding the phrase "(contains glutamate)" to the common or usual names of certain protein hydrolysates that contain substantial amounts of glutamate.[6] For example, if the proposal were adopted, hydrolyzed soy protein would have to be declared on food labels as "hydrolyzed soy protein (contains glutamate)."
Asia
The INTERMAP Cooperative Research Group conducted a study of 752 healthy Chinese (48.7% women), aged 40-59 years, randomly sampled from three rural villages in north and south China and determined that MSG intake may be positively related to increased BMI (Body Mass Index)[7]
Australia and New Zealand
Standard 1.2.4 of the Australia New Zealand Food Standards Code requires the presence of MSG as a food additive to be labeled. The label must bear the food additive class name (e.g. flavour enhancer), followed by either the name of the food additive (e.g. MSG) or its International Numbering System (INS) number (e.g. 621).
See also
- Ajinomoto Co., Inc.
- Umami
- Excitotoxicity
- Flavour enhancer
- Disodium glutamate
- List of food additives
- Yeast extract
References
- ↑ Kawakita, Tetsuya; Sano, Chiaki; Shioya, Shigeru; Takehara, Masahiro; Yamaguchi, Shizuko (2005). "Monosodium Glutamate". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. DOI:711 10.1002/14356007.a16 711.
- ↑ "Production process". Encyclopedia of Amino Acids. Anjimoto Co., Inc.
- ↑ http://www.ajinomoto.co.jp/ajinomoto/A-Company/company/zaimu/pdf/fact/food_biz.pdf
- ↑ Moskin, Julia (2008-03-05). "Yes, MSG, the Secret Behind the Savor", New York Times.
- ↑ 5.0 5.1 Rundlett, Kimber L; Armstrong, Daniel W (1994). "Evaluation of free D-glutamate in processed foods". Chirality 6 (4): pp. 277–282. doi:. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7915127&dopt=Abstract.
- ↑ 6.0 6.1 "FDA and Monosodium Glutamate (MSG)". United States Food and Drug Administration. United States Department of Health and Human Services (1995-08-31).
- ↑ He, Ka; Zhao, Liancheng; Daviglus, Martha L; Dyer, Alan R; Van Horn, Linda; Garside, Daniel; Zhu, Linguang; Dongshuang, Guo; Wu, Yangfeng; Zhou, Beifan; Stamler, Jeremiah (August 2008). "Association of monosodium glutamate intake with overweight in Chinese adults: the INTERMAP Study". Obesity (The Obesity Society) 16 (8): pp. 1875–1880. doi:. PMID 18497735. http://www.nature.com/oby/journal/v16/n8/abs/oby2008274a.html.