Mifepristone
 |
|
|
Mifepristone
|
|
| Systematic (IUPAC) name | |
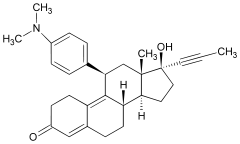
| 11β-[p-(Dimethylamino)phenyl]- 17β-hydroxy-17-(1-propynyl)estra- 4,9-dien-3-one |
|
| Identifiers | |
| CAS number | |
| ATC code | G03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | C29H35NO2 |
| Mol. mass | 429.60 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 69% |
| Metabolism | hepatic |
| Half life | 18 hours |
| Excretion | Fecal: 83%; Renal: 9% |
| Therapeutic considerations | |
| Pregnancy cat. |
X(US) Used for terminating pregnancy |
| Legal status |
℞-only(US) |
| Routes | Oral |
Mifepristone is a synthetic steroid compound used as a pharmaceutical. It is used as an abortifacient in the first two months of pregnancy, and in smaller doses as an emergency contraceptive. During early trials, it was known as RU-486, its designation at the Roussel Uclaf company, which designed the drug. The drug was initially made available in France, and other countries then followed—often amid controversy. In France and countries other than the United States it is marketed and distributed by Exelgyn Laboratories under the tradename Mifegyne. In the United States it is sold by Danco Laboratories under the tradename Mifeprex.
Contents |
History
The compound was discovered by researchers at Roussel Uclaf of France in 1980 (the "RU" in RU-486) while they were studying glucocorticoid receptor antagonists. Clinical testing began in 1982. The drug was first licensed in France in 1988, for use in combination with a prostaglandin, under the name Mifegyne. After license approval but before market release, Roussel Uclaf announced it would abandon distribution of the drug, bowing to pressure from pro-life groups and the threat of a boycott. However, two days later, the French government, part owner of Roussel Uclaf, intervened, leading to the resumption of production and distribution of RU-486. The French Health Minister, explaining the government's intervention, stated, "I could not permit the abortion debate to deprive women of a product that represents medical progress. From the moment Government approval for the drug was granted, RU-486 became the moral property of women."[1]
Indications
Mifegyne is sold outside the U.S. by Exelgyn Laboratories, made in France, and is approved for:
- Medical termination of intrauterine pregnancies of up to 49 days gestation (up to 63 days gestation in Britain and Sweden)
- Softening and dilatation of the cervix prior to mechanical cervical dilatation for pregnancy termination
- Use in combination with gemeprost for termination of pregnancies between 13 and 24 weeks gestation
- Labor induction in fetal death in utero.[2]
Mifeprex is sold in the U.S. by Danco Laboratories, made in China,[3] and is FDA-approved in the U.S. to terminate intrauterine pregnancies of up to 49 days gestation. Under the FDA-approved regimen, a 600 mg dose is administered by a clinician following a counseling session. Two days later, a clinician administers 400 µg of another medicine, misoprostol, to induce contractions. In European studies, this method terminated 96 to 99% of pregnancies of up to 49 days gestation, but in one large multicenter trial in the U.S. conducted from September 1994 to September 1995, the efficacy was lower (92%), which the authors of the study suggested may have been due to lack of experience with this method in the U.S. and/or the design of their study.[4] In Europe and China, an observation period of several hours is required after administration of misoprostol. If expulsion of fetal tissue does not occur during the observation period, surgical abortion is offered. There is no required observation period in the U.S., but it is strongly recommended.[5]
According to the current RCOG abortion evidence-based clinical guideline:[6]
- All methods of first-trimester abortion carry a small risk of failure to terminate the pregnancy, thus necessitating a further procedure. The risk for surgical abortion is around 0.23% and for medical abortion between 0.1% and 1.4% (depending on the regimen used and the experience of the centre).
- Medical abortion using mifepristone plus prostaglandin is the most effective method of abortion at gestations of less than 7 weeks.
- Conventional vacuum aspiration should be avoided at gestations below 7 weeks.
- Early vacuum aspiration using a rigorous protocol (which includes magnification of aspirated material and indications for serum βhCG follow-up) may be used at gestations below 7 weeks, although data suggest that the failure rate is higher than for medical abortion.
- Medical abortion using mifepristone plus prostaglandin continues to be an appropriate method for women in the 7–9 week gestation band.
Mifepristone can also be used in smaller doses as an emergency contraceptive; if taken after sex but before ovulation, it can prevent ovulation and so prevent pregnancy. In this role, a 10 mg dose is not as effective as the 600 mg dose, but has fewer side-effects.[7] Mifeprex and Mifegyne are only available in 200 mg tablets.[8] A review of studies in humans found that the contraceptive effects of the 10 mg dose were probably due mainly to its effects on ovulation, and not inhibition of implantation, but "the knowledge of the mechanism of action remains incomplete." Treatment with 200 mg of mifepristone changes steroid receptor expression in the fallopian tube, inhibits endometrial development, and effectively prevents implantation.[9]
Other uses
Other medical applications of mifepristone that have been studied in Phase II clinical trials include regular long-term use as an oral contraceptive, and treatment of: uterine fibroids, endometriosis, major depression with psychotic features, glaucoma, meningiomas, breast cancer, ovarian cancer, prostate cancer, and some types of Cushing's syndrome.
Mifepristone has been studied as an antiretroviral for its in vivo interference with the HIV regulatory protein vpr. It showed no detectable anti-HIV activity in clinical trials.[10] [11][12][13] It is currently being studied as a treatment for chronic multisymptom illness.[14] Mifepristone has not been approved by the FDA for any of these uses.
Contraindications
In clinical trials, nearly all women using mifepristone experienced abdominal pain, uterine cramping, and vaginal bleeding or spotting for an average of 9–16 days. Up to 8% of women experienced some type of bleeding for 30 days or more. Other less common side effects included nausea, vomiting, diarrhea, dizziness, fatigue, and fever.[15] Pelvic inflammatory disease (PID) is a very rare but serious complication.[16] Excessive bleeding and incomplete termination of a pregnancy require further intervention by a doctor (such as vacuum aspiration). Between 4.5 and 7.9% of women required surgical intervention in clinical trials.[15] Mifepristone is contraindicated in the presence of an intrauterine device (IUD), as well as with ectopic pregnancy, adrenal failure, hemorrhagic disorders, inherited porphyria, and anticoagulant or long-term corticosteroid therapy.[15]
The FDA prescribing information states that there are no data on the safety and efficacy of mifepristone in women with chronic medical conditions, and that "women who are more than 35 years of age and who also smoke 10 or more cigarettes per day should be treated with caution because such patients were generally excluded from clinical trials of mifepristone."[15]
Adverse effects
No long-term studies to evaluate the carcinogenic potential of mifepristone have been performed. Results from studies conducted in vitro and in animals have revealed no genotoxic potential for mifepristone. A preliminary study in mice indicates that a single dose of mifepristone inhibited apoptosis of liver cells in the treated animals, which the authors hypothesized might increase susceptibility to hepatic cancer.[17]
Neonatal exposure to a single large dose of mifepristone in rats was not associated with any reproductive problems, although chronic low-dose exposure of newborn rats to mifepristone was associated with structural and functional reproductive abnormalities.[15]
Teratology studies in mice, rats and rabbits revealed teratogenicty for rabbits, but not rats or mice.[15] The rate of birth defects in human infants exposed in utero to mifepristone and misoprostol is very low,[18] and may be due to misoprostol alone.[19]
Pharmacology
Mifepristone is a 19-nor steroid with a bulky p-(dimethylamino)phenyl substituent above the plane of the molecule at the 11β-position responsible for inducing or stabilizing an inactive receptor conformation and a hydrophobic 1-propynyl substituent below the plane of the molecule at the 17α-position that increases its progesterone receptor binding affinity. In the presence of progesterone, mifepristone acts as a competitive receptor antagonist at the progesterone receptor (in the absence of progesterone, mifepristone acts as a partial agonist).[20][11][21]
In addition to being an antiprogestogen, mifepristone is also an antiglucocorticoid and a weak antiandrogen. Mifepristone's relative binding affinity at the progesterone receptor is more than twice that of progesterone, its relative binding affinity at the glucocorticoid receptor is more than three times that of dexamethasone and more than ten times that of cortisol, its relative binding affinity at the androgen receptor is less than one third that of testosterone. It does not bind to the estrogen receptor or the mineralocorticoid receptor.[22]
Mifepristone as a regular contraceptive at 2 mg daily prevents ovulation (1 mg daily does not). A single preovulatory 10 mg dose of mifepristone delays ovulation by 3 to 4 days and is as effective an emergency contraceptive as a single 1.5 mg dose of the progestin levonorgestrel.[12]
In women, mifepristone at doses greater or equal to 1 mg/kg antagonizes the endometrial and myometrial effects of progesterone. In humans, an antiglucocorticoid effect of mifepristone is manifested at doses greater or equal to 4.5 mg/kg by a compensatory increase in ACTH and cortisol. In animals, a weak antiandrogenic effect is seen with prolonged administration of very high doses of 10 to 100 mg/kg.[2][23]
In medical abortion regimens, mifepristone blockade of progesterone receptors directly causes endometrial decidual degeneration, cervical softening and dilatation, release of endogenous prostaglandins and an increase in the sensitivity of the myometrium to the contractile effects of prostaglandins. Mifepristone induced decidual breakdown indirectly leads to trophoblast detachment, resulting in decreased syncytiotrophoblast production of hCG, which in turn causes decreased production of progesterone by the corpus luteum (pregnancy is dependent on progesterone production by the corpus luteum through the first 9 weeks of gestation--until placental progesterone production has increased enough to take the place of corpus luteum progesterone production). When followed sequentially by a prostaglandin, mifepristone 200 mg is (100 mg may be, but 50 mg is not) as effective as 600 mg in producing a medical abortion.[20][21]
Legal status
United States
Roussel Uclaf did not seek U.S. approval, so U.S. availability was not an initial possibility.[24] The first Bush administration banned importation of Mifepristone for personal use in 1989, a decision supported by Roussel Uclaf. In 1994, Roussel Uclaf gave the U.S. drug rights to the Population Council in exchange for immunity from any product liability claims.[25][26] The Population Council sponsored US clinical trials.[27] The drug went on approvable status from 1996. Production was intended to begin through the Danco Group in 1996 but they withdrew briefly in 1997 due to a corrupt business partner, delaying availability again.[28][29] Mifepristone was approved for abortion in the U.S. by the FDA, in September 2000, during the final months of President Bill Clinton's administration.[30] It is legal and available in all 50 states, Washington D.C., Guam and Puerto Rico.[31] Medical abortions as a percentage of total abortions in the United States have increased every year since the approval of mifepristone: 1.0% in 2000, 2.9% in 2001, 5.2% in 2002, 7.9% in 2003, 9.3% in 2004 (14.2% of those less than 9 weeks gestation); although data is limited by eleven states not reporting statistics to the Centers for Disease Control and Prevention (CDC) (including California where an estimated >23% of total U.S. abortions were performed in 1997).[32]
Subsection H
Some drugs are approved by the FDA under sub-section H, which has two sub-parts. The first sets forth ways to rush experimental drugs, such as aggressive HIV and cancer treatments, to market when speedy approval is deemed vital to the health of potential patients. The second part of sub-section H applies to drugs that not only must meet restrictions for use due to safety requirements, but also are required to meet postmarketing surveillance to establish that the safety results shown in clinical trials are seconded by use in a much wider population. Mifepristone was approved under the second part of sub-section H. The result is that women cannot pick the drug up at a pharmacy but must now receive it directly from a doctor. Due to the possibility of adverse reactions such as excessive bleeding which may require a blood transfusion and incomplete abortion which may require surgical intervention, the drug is only considered safe if a physician who is capable of administering a blood transfusion or a surgical abortion is available to the patient in the event of such emergencies.[33] The approval of mifepristone under Subsection H included a black box warning.
FDA controversy
Many pro-life groups in the US actively campaigned against the approval of mifepristone,[34][35][36] and continue to actively campaign for its withdrawal.[37] They cite either ethical issues with abortion or safety concerns regarding the drug and the adverse reactions associated with it, including death.[38] The proposed "RU-486 Suspension and Review Act," also known as Holly's Law, was initiated by a citizen's petition to the FDA from namesake Holly Patterson's father,[39] and the May 17, 2006 House Subcommittee on Criminal Justice, Drug Policy and Human Resources hearing entitled "RU-486 - Demonstrating a Low Standard for Women’s Health?”[40]—called by its pro-life chairman Rep. Mark Souder—are the principal results of this effort. Religious and pro-life groups outside the US have also protested mifepristone, especially in Germany[41] and Australia.[42][43]
A small but vocal group of female scientists from the Massachusetts Institute of Technology's Institute on Women and Technology issued a report under the name of "Feminist International Network of Resistance to Reproductive and Genetic Engineering" in the early '90s to express their opposition to mifepristone, because "We felt what was being lost in the political debate was how the drug affects women. In contrast with the groups who are anti-feminist and anti-abortion, the Institute on Women and Technology advocates women's rights to abortion and self determination," said Dr. Janice Raymond of FINRRAGE. Additional feminist critics exist, such as Pauline Connor (LI.B.) of Feminists Against Eugenics in England who stated, "What has been presented as a simple, pill-popping exercise is, in fact, an intensely medicalized and painful procedure which can involve up to four clinic visits and last 12 days."[44]
Europe
Mifepristone was approved for use in France in 1988 (initial marketing in 1989), the United Kingdom in 1991, Sweden in 1992, then Austria, Belgium, Denmark, Finland, Germany, Greece, Luxembourg, the Netherlands, Spain, and Switzerland in 1999.[45] In 2000, it was approved in Norway. Serbia and Montenegro approved it in 2001,[46] Latvia in 2002, Estonia in 2003, Albania and Hungary in 2005.[47] In Sweden and the UK, mifepristone is licensed for use with vaginal gemeprost instead of oral misoprostol. As of seven years ago, more than 620,000 women in Europe had had medical abortions using a mifepristone regimen.[48] In France, the percentage of medical abortions continues to increase, from 38% of all abortions in 2003 to 42% of all abortions in 2004.[49] In England and Wales, 42% of early abortions (less than 9 weeks gestation) in 2006 were medical; the percentage of all abortions that are medical has increased every year for the past 11 years (from 5% in 1995 to 30% in 2006).[50] In Scotland, 77.8% of early abortions (less than 10 weeks gestation) in 2006 were medical; the percentage of all abortions that are medical has increased every year for the past 14 years (from 16.4% in 1992 to 59.1% in 2006).[51] In Sweden, 60.6% of early abortions (before the end of the 12th week of gestation) in 2006 were medical; 56.3% of all abortions in 2006 were medical.[52] In Denmark, mifepristone was used in between 3,000 and 4,000 of just over 15,000 abortions in 2005.[53] Mifepristone is not approved in Ireland, where abortion is illegal, or Poland, where abortion is highly restricted.[54] Clinical trials in Italy have been constrained by protocols requiring women be hospitalized for three days.[55] It was approved in Hungary in 2005, but as of 2005 had not been released on the market yet, and was the target of protests.[56]
Other countries
Mifepristone was banned in Australia in 1996. In late 2005, a Private Member's bill was introduced to the Australian Senate to lift the ban and transfer the power of approval to the Therapeutic Goods Administration (TGA). The move caused much debate in the Australian media and amongst politicians. The Bill passed the Senate on 10 February 2006, and whilst mifepristone is now legal for use in Australia, as of yet, no drug company has applied to import and distribute it. Currently there are only a couple of known instances where a doctor has applied to the TGA for dispensing mifepristone in specific cases. In New Zealand, pro-choice doctors established an import company, Istar, and submitted a request for approval to MedSafe, the New Zealand pharmaceutical regulatory agency. After a court case brought by Right to Life New Zealand failed, use of mifepristone was permitted.[57]
The drug was approved in Israel in 1999.[58]
Clinical trials of mifepristone in China began in 1985. In October 1988, China became the first country in the world to approve mifepristone. Chinese organizations tried to purchase mifepristone from Roussel Uclaf, but they refused to sell it to them, so in 1992 China began its own domestic production of mifepristone. In 2000, the cost of medical abortion with mifepristone was higher than surgical abortion and the percentage of medical abortions varied greatly, ranging from 30% to 70% in cities to being almost nonexistent in rural areas.[59][60] A report from the United States Embassy in Beijing in 2000 said mifepristone had been widely used in Chinese cites for about two years, and that according to press reports a black market had developed with many women starting to it illegally (without a prescription) from private clinics and drugstores for about $15, causing Chinese authorities to worry about medical complications from use without physician supervision.[61]
In 2001, mifepristone was approved in Taiwan.[62] Vietnam included mifepristone in the National Reproductive Health program in 2002.[63]
It is approved in only one subsaharan African country--South Africa, where it was approved in 2001.[64] It is also approved in one north African country--Tunisia, also in 2001.[65]
Mifepristone was approved for use in India in 2002, where medical abortion is referred to as "Medical Termination of Pregnancy" (MTP). It is only available under medical supervision, not by prescription, due to adverse reactions such as excessive bleeding, and there are criminal penalties for buying or selling it on the black market or over-the-counter at pharmacies.[66]
Medical abortion is available in Canada on a limited basis using methotrexate and misoprostol; mifepristone is not legally approved, and importation of that drug in Canada is currently illegal. Clinical trials were done in 2000 in various Canadian cities comparing methotrexate to mifepristone, after approbation by the federal government. While both drugs had overall similar results, mifepristone was found to act faster. [67] As of May 2005, it is unclear whether or when RU-486 will be approved for use in Canada.[68]
Mifepristone was registered for use in Russia and Ukraine in 2000, and in Azerbaijan, Belarus, Georgia, and Uzbekistan in 2002, Moldova in 2004, and Armenia in 2007.[69][47]
Notes and references
- ↑ Julie A. Hogan (2000). "The Life of the Abortion Pill in the United States". Legal Electronic Document Archive, Harvard Law School. Retrieved on 2006-09-14.
- ↑ 2.0 2.1 Exelgyn Laboratories (February 2006). "Mifegyne UK Summary of Product Characteristics (SPC)". Retrieved on 2007-03-09.
- ↑ "Chinese to Make RU-486 for U.S.". Washingtonpost.com (2000). Retrieved on 2006-08-22.
- ↑ Spitz IM, Bardin CW, Benton L, Robbins A (1998). "Early pregnancy termination with mifepristone and misoprostol in the United States". N Engl J Med 338 (18): 1241–7. doi:. PMID 9562577.
- ↑ Suzanne Daley (October 5, 2000). "Europe Finds Abortion Pill is No Magic Cure-All". The New York Times. Retrieved on 2006-09-16.
- ↑ RCOG (2004) (PDF). The Care of Women Requesting Induced Abortion : Evidence-based clinical guideline number 7. London: RCOG Press. ISBN 1-904752-06-3. http://www.rcog.org.uk/resources/Public/pdf/induced_abortionfull.pdf.
- ↑ Piaggio G et al (2003). "Meta-analysis of randomized trials comparing different doses of mifepristone in emergency contraception". Contraception 68 (6): 447. doi:. PMID 14698075.
- ↑ Wertheimer, Randy E. (2000-11-15). "Emergency Postcoital Contraception" (HTML). American Family Physician (American Academy of Family Physicians). http://www.aafp.org/afp/20001115/2287.html. Retrieved on 2006-07-23.
- ↑ Gemzell-Danielsson, K.; Marions, L. (2004-06-10). "Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception" (HTML). Human Reproduction Update (Oxford University Press) 10 (4): 341–348. doi:. PMID 15192056.
- ↑ Flexner, Charles. “HIV drug development: the next 25 years”. Nat Rev Drug Discov. 2007 Dec;6(12):959-66.
- ↑ 11.0 11.1 Schimmer, Bernard P.; Parker, Keith L. (2006). "Adrenocorticotropic Hormone; Adrenocortical Steroids and Their Synthetic Analogs; Inhibitors of the Synthesis and Actions of Adrenocortical Hormones". in in Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed. ed.). New York: McGraw-Hill. pp. pp. 1587-1612. ISBN 0-07-142280-3.
- ↑ 12.0 12.1 Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM (2005). "Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications". Hum Reprod Update 11 (3): 293–307. doi:. PMID 15790602.
- ↑ Tang OS, Ho PC (2006). "Clinical applications of mifepristone". Gynecol Endocrinol 22 (12): 655–9. doi:. PMID 17162706.
- ↑ "A Controlled Trial of Mifepristone in Gulf War Veterans With Chronic Multisymptom Illness". ClinicalTrials.gov. U.S. National Institutes of Health (June 2008). Retrieved on November 7, 2008.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 "Mifeprex label" (PDF). FDA (2005-07-19). Retrieved on 2006-08-22.
- ↑ Lawton, BA et al (2006). "Atypical presentation of serious pelvic inflammatory disease following mifepristone-induced abortion". Contraception 73 (4): 431. doi:. PMID 16531180.
- ↑ Youssef, Jihan and Badr, Mostafa (2003). "Hepatocarcinogenic potential of the glucocorticoid antagonist RU486 in B6C351 mice". Molecular Cancer. Retrieved on 2006-08-26.
- ↑ Margaret M. Gary and Donna J. Harrison (December 2005). "Analysis of Severe Adverse Events Related to the Use of Mifepristone as an Abortifacient". The Annals of Pharmacology. Retrieved on 2006-09-14.
- ↑ Orioli, IM and Castilla, EE (2000). "Epidemiological assessment of misoprostol teratogenicity". BJOG 107 (4): 519–23. doi:. PMID 10759272.
- ↑ 20.0 20.1 Loose, Davis S.; Stancel, George M. (2006). "Estrogens and Progestins". in in Brunton, Laurence L.; Lazo, John S.; Parker, Keith L. (eds.). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed. ed.). New York: McGraw-Hill. pp. pp. 1541-1571. ISBN 0-07-142280-3.
- ↑ 21.0 21.1 Fiala C, Gemzel-Danielsson K (2006). "Review of medical abortion using mifepristone in combination with a prostaglandin analogue". Contraception 74 (1): 66–86. doi:. PMID 16781264.
- ↑ Heikinheimo O, Kekkonen R, Lahteenmaki P (2003). "The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action". Contraception 68 (6): 421–6. doi:. PMID 14698071.
- ↑ Danco Laboratories (July 19, 2005). "Mifeprex US prescribing information". Retrieved on 2007-03-09.
- ↑ Klitsch M (Nov-December 1991). "Antiprogestins and the abortion controversy: a progress report". Fam Plann Perspect 23 (6): 275–82. doi:. PMID 1786809.
- ↑ Katharine Q. Seelye (1997-01-05-17). "Accord Opens Way for Abortion Pill in US in 2 Years". Retrieved on 2006-08-26.
- ↑ Nancy Gibbs (October 2, 2000). "The Pill Arrives". Cnn.com. Retrieved on 2006-09-20.
- ↑ Tamar Lewin (January 30, 1995). "Clinical Trials Giving Glimpse of Abortion Pill". The New York Times. Retrieved on 2006-09-20.
- ↑ Tamar Lewin (November 13, 1997). "Lawsuits' Settlement Brings New Hope for Abortion Pill". The New York Times. Retrieved on 2006-09-16.
- ↑ Sharon Lerner (August 2000). "RU Pissed Off Yet?". Village Voice. Retrieved on 2006-09-16.
- ↑ "FDA approval letter for Mifepristone". U.S. Gov (September 28, 2000). Retrieved on 2006-09-16.
- ↑ "Medication Abortion in the United States: Mifepristone Fact Sheet". Gynuity Health Projects (2005).
- ↑ Strauss LT, Gamble SB, Parker WY, Cook DA, Zane SB, Hamdan S; Centers for Disease Control and Prevention (CDC) (2007). "Abortion surveillance--United States, 2004". MMWR Surveill Summ 56 (9): 1–33. PMID 18030283. http://www.cdc.gov/mmwr/pdf/ss/ss5609.pdf. Table 18 (39 states and NYC).
- ↑ Woodcock, Janet (2006-05-12). "Testimony on RU-486". Committee on Government Reform, House of Representatives. FDA. Retrieved on 2006-08-19.
- ↑ Paige Comstock Cunningham, Leanne McCoy, Clarke D. Ferguson (February 28, 1995). "Citizen Petition to the U.S. Food and Drug Administration". Americans United for Life. Retrieved on 2006-09-20.
- ↑ Margaret Talbot (July 11, 1999). "The Little White Bombshell". The New York Times. Retrieved on 2006-09-20.
- ↑ "Abortion Foes To Boycott Drugs (Altace) Made By RU-486 Manufacturer". The Virginia Pilot (July 8, 1994). Retrieved on 2006-09-15.
- ↑ Stan Guthrie (June 11, 2001). "Counteroffensive Launched on RU-486". Christianity Today. Retrieved on 2006-09-20.
- ↑ Gina Kolata (September 24, 2003). "Death at 18 Spurs Debate Over a Pill For Abortion". The New York Times. Retrieved on 2006-09-20.
- ↑ Karen Tumulty (October 14, 2002). "Jesus and the FDA". Time. Retrieved on 2006-09-20.
- ↑ "RU-486 - Demonstrating a Low Standard for Women’s Health?". House Subcommittee on Criminal Justice, Drug Policy and Human Resources (2006-05-17). Retrieved on 2006-08-25.
- ↑ John L. Allen (February 12, 1999). "Abortion debates rock Germany: introduction of abortion pill exacerbates controversy". National Catholic Reporter. Retrieved on 2006-09-14.
- ↑ "Catholic and Evangelical students join Muslims in RU-486 fight". Catholic News (February 9, 2006). Retrieved on 2006-09-18.
- ↑ "Death Toll Rises to 11 Women". Australians Against RU-486 (2006). Retrieved on 2006-09-20.
- ↑ Annette MacDonald (1992). "RU-486: A Dangerous Drug". The Vancouver Sun. Retrieved on 2006-08-22.
- ↑ Christin-Maitre S, Bouchard P, Spitz IM (2000). "Medical termination of pregnancy". N Engl J Med 342 (13): 946–56. doi:. PMID 10738054.
- ↑ Stojnic J et al (2006). Medicamentous abortion with mifepristone and misoprostol in Serbia and Montenegro. 63. pp. 558–63. PMID 16796021.
- ↑ 47.0 47.1 "Mifepristone approval" (PDF). Gynuity.org accessdate=2007-07-27 (2007).
- ↑ "FDA Approves Mifepristone for the Termination of Early Pregnancy". FDA press release/US Gov (2000). Retrieved on 2006-09-17.
- ↑ Vilain, Annick (2006). "Voluntary terminations of pregnancies in 2004" (PDF). DREES, Ministry of Health. Retrieved on 2007-02-18.
- ↑ Department of Health (2007). "Abortion statistics, England and Wales: 2006". Retrieved on 2007-07-08.
- ↑ ISD Scotland (2007). "Abortion Statistics 2006". Retrieved on 2007-07-08.
- ↑ National Board of Health and Welfare, Sweden (2007). "Induced Abortions 2006" (PDF). Retrieved on 2007-07-08.
- ↑ "The abortion pill Mifegyne tested for adverse reactions". Danish Medicines Agency (July 27, 2005). Retrieved on 2006-09-20.
- ↑ Peter S. Green (June 24, 2003). "A Rocky Landfall for a Dutch Abortion Boat". The New York Times. Retrieved on 2006-09-16.
- ↑ Rey, Anne-Marie Rey (October 17, 2006). "Abortion in Italy". SVSS-USPDA. Retrieved on 2007-03-01.
- ↑ "Abortion pill sparks bitter protest". The Budapest Times (September 19, 2005). Retrieved on 2006-09-16.
- ↑ Sparrow MJ (2004). "A woman's choice". Aust NZ J Obstet Gynaecol 44 (2): 88. doi:. PMID 15089829.
- ↑ Etienne-Emile Baulieu, Daniel S. Seidman, Selma Hajri (October 2001). "Mifepristone(RU-486) and voluntary termination of prgnancy: enigmatic variations or anecdotal religion-based attitudes?". Human Reproduction. Retrieved on 2006-09-16.
- ↑ Ulmann A (2000). "The development of mifepristone: a pharmaceutical drama in three acts". J Am Med Women's Assoc 55 (3 Suppl): 117–20. PMID 10846319.
- ↑ Wu S (2000). "Medical abortion in China". J Am Med Women's Assoc 55 (3 Suppl): 197–9, 204. PMID 10846339.
- ↑ "Family planning in China: RU-486, abortion, and population trends". U.S. Embassy Beijing (2000). Retrieved on 2006-09-14.
- ↑ Tsai EM, Yang CH, Lee JN (2002). "Medical abortion with mifepristone and misoprostol: a clinical trial in Taiwanese women". J Formos Med Assoc 101 (4): 277–82. PMID 12101864.
- ↑ Ganatra B, Bygdeman M, Nguyen DV, Vu ML, Phan BT (2004). "From research to reality: the challenges of introducing medical abortion into service delivery in Vietnam". Reprod Health Matters 12 (24): 105. doi:. PMID 15938163.
- ↑ "Medical Abortion-Implications for Africa". Ipas (2003). Retrieved on 2006-09-16.
- ↑ Hajri S (2004). "Medication abortion: the Tunisian experience". Afr J Reprod Health 8 (1): 63–9. doi:. PMID 15487615.
- ↑ "Mifepristone can be sold only to approved MTP Centres: Rajasthan State HRC". Indian Express Health Care Management (2000).
- ↑ ."Results of the Canadian trials of RU486, the 'Abortion Pill'." (n.d.). Retrieved 2006-12-08.
- ↑ Jennifer Laliberte (September 30, 2005). "Still no mifepristone for Canada: is it safe?". National Review of Medicine. Retrieved on 2006-09-16.
- ↑ "Medication Abortion". Ibis (2002). Retrieved on 2006-09-19.
See also
|
External links
- US Food and Drug Administration Mifeprex (mifepristone) Information
- Commonly asked questions about RU-486 from the education arm of the National Coalition of Abortion Providers
- Danco prescribing information
- Australians for RU-486 - established in February 2006 to lobby for passage of bill in Australia's Parliament to enable the availability of Mifepristone
|
||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||