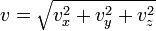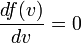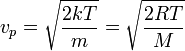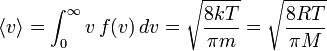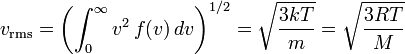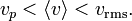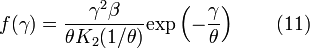Maxwell–Boltzmann distribution
The Maxwell–Boltzmann distribution is a probability distribution with applications in physics and chemistry. The most common application is in the field of statistical mechanics. The temperature of any (massive) physical system is the result of the motions of the molecules and atoms which make up the system. These particles have a range of different velocities, and the velocity of any single particle constantly changes due to collisions with other particles. However, the fraction of a large number of particles within a particular velocity range is nearly constant. The Maxwell distribution of velocities specifies this fraction, for any velocity range, as a function of the temperature of the system. It is named after James Clerk Maxwell and Ludwig Boltzmann.
The distribution can be thought of as the magnitude of a 3-dimensional vector whose components are independent and normally distributed with mean 0 and standard deviation  . If
. If 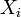 are distributed as
are distributed as 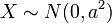 , then
, then
is distributed as a Maxwell–Boltzmann distribution with parameter  .
.
Contents |
Physical applications of the Maxwell–Boltzmann distribution
The Maxwell–Boltzmann distribution forms the basis of the kinetic theory of gases, which explains many fundamental gas properties, including pressure and diffusion. The Maxwell–Boltzmann distribution is usually thought of as the distribution of molecular speeds in a gas, but it can also refer to the distribution of velocities, momenta, and magnitude of the momenta of the molecules, each of which will have a different probability distribution function, all of which are related.
The Maxwell–Boltzmann distribution can be derived using statistical mechanics (see Maxwell–Boltzmann statistics). It corresponds to the most probable speed distribution in a collisionally-dominated system consisting of a large number of non-interacting particles in which quantum effects are negligible. Since interactions between the molecules in a gas are generally quite small, the Maxwell-Boltzmann distribution provides a very good approximation of the conditions in a gas.
There are many cases (such as elastic collisions) where these conditions do not apply. For example, in the physics of the ionosphere and space plasmas recombination and collisional excitation (i.e. radiative processes) are important, especially for electrons. Applying the Maxwell distribution and its assumptions in this case, you would get the wrong numbers, and miss the basic physics of the problem. Another example where applying the Maxwell-Boltzmann Distribution would give incorrect results is in cases where the quantum thermal wavelength of the gas is not small compared to the distance between particles. There, the theory would fail to account for significant quantum effects. Also, as it is based on nonrelativistic assumptions, the Maxwell-Boltzmann distribution does not predict zero probability for molecular velocities in excess of the speed of light.
Derivation
The original derivation by Maxwell assumed all three directions would behave in the same fashion, but a later derivation by Boltzmann dropped this assumption using kinetic theory. The Maxwell–Boltzmann distribution can now most readily be derived from the Boltzmann distribution for energies:
where Ni is the number of molecules at equilibrium temperature T, in a state i which has energy Ei and degeneracy gi, N is the total number of molecules in the system and k is the Boltzmann constant. (Note that sometimes the above equation is written without the degeneracy factor gi. In this case the index i will specify an individual state, rather than a set of gi states having the same energy Ei.) Because velocity and speed are related to energy, Equation 1 can be used to derive relationships between temperature and the speeds of molecules in a gas. The denominator in this equation is known as the canonical partition function.
Distribution of the momentum vector
What follows is a derivation wildly different from the derivation described by James Clerk Maxwell and later described with fewer assumptions by Ludwig Boltzmann. Instead it is close to Boltzmann's later approach of 1877.
For the case of an "ideal gas" consisting of non-interacting atoms in the ground state, all energy is in the form of kinetic energy. The relationship between kinetic energy and momentum for massive particles is
where p2 is the square of the momentum vector p = [px, py, pz]. We may therefore rewrite Equation 1 as:
where Z is the partition function, corresponding to the denominator in Equation 1. Here m is the molecular mass of the gas, T is the thermodynamic temperature and k is the Boltzmann constant. This distribution of Ni/N is proportional to the probability density function fp for finding a molecule with these values of momentum components, so:
The normalizing constant c, can be determined by recognizing that the probability of a molecule having any momentum must be 1. Therefore the integral of equation 4 over all px, py, and pz must be 1.
It can be shown that:
Substituting Equation 5 into Equation 4 gives:
The distribution is seen to be the product of three independent normally distributed variables 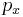
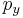 , and
, and 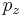 , with variance
, with variance 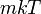 . Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with
. Additionally, it can be seen that the magnitude of momentum will be distributed as a Maxwell–Boltzmann distribution, with 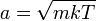 .
.
Distribution of the energy
Using p² = 2mE, and the distribution function for the MAGNITUDE of the momentum (see section on distribution of speeds below), we get the energy distribution:
Since the energy is proportional to the sum of the squares of the three normally distributed momentum components, this distribution is a chi-square distribution with three degrees of freedom:
where
The Maxwell–Boltzmann distribution can also be obtained by considering the gas to be a quantum gas.
Distribution of the velocity vector
Recognizing that the velocity probability density fv is proportional to the momentum probability density function by
and using p = mv we get
which is the Maxwell–Boltzmann velocity distribution. The probability of finding a particle with velocity in the infinitesimal element [dvx, dvy, dvz] about velocity v = [vx, vy, vz] is
Like the momentum, this distribution is seen to be the product of three independent normally distributed variables 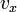
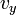 , and
, and 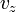 , but with variance
, but with variance 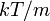 . It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity [vx, vy, vz] is the product of the distributions for each of the three directions:
. It can also be seen that the Maxwell–Boltzmann velocity distribution for the vector velocity [vx, vy, vz] is the product of the distributions for each of the three directions:
where the distribution for a single direction is
This distribution has the form of a normal distribution, with variance 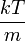 . As expected for a gas at rest, the average velocity in any particular direction is zero.
. As expected for a gas at rest, the average velocity in any particular direction is zero.
Distribution of speeds

Usually, we are more interested in the speeds of molecules rather than their component velocities. The Maxwell-Boltzmann distribution of speeds is written as
where speed, v, is defined as
Note that the units of f(v) in equation are probability per speed, or just reciprocal speed as in the graph at the right.
Since the speed is the square root of the sum of squares of the three independent, normally distributed velocity components, this distribution is a Maxwell–Boltzmann distribution, with 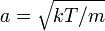 .
.
We are often more interested in quantities such as the average speed of the particles rather than the actual distribution. The mean speed, most probable speed (mode), and root-mean-square can be obtained from properties of the Maxwell–Boltzmann distribution.
Typical speeds
Although the above equation gives the distribution of speeds or in other words the fraction of molecules having a particular speed, we are often more interested in quantities such as the average speed of the particles rather than the actual distribution.
The most probable speed, vp, is the speed most likely to be possessed by any molecule in the system and corresponds to the maximum value or mode of f(v). To find it, we calculate df/dv, set it to zero and solve for v:
which yields:
Where R is the gas constant and M = NA m is the molar mass of the substance.
For nitrogen (the primary component of air) at room temperature (~300 K), this gives 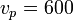 m/s
m/s
The mean speed is the mathematical average of the speed distribution
The root mean square speed, vrms is the square root of the average squared speed:
The typical speeds are related as follows:
Distribution of speeds for a relativistic gas

As the gas becomes hotter and kT approaches or exceeds mc2, the speed distribution of this relativistic Maxwellian gas is given by the Maxwell-Juttner distribution[1]:
where 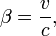
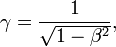
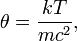 and
and 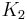 is the modified Bessel function of the second kind.
is the modified Bessel function of the second kind.
See also
- Boltzmann factor
- Rayleigh distribution
- Ideal gas law
- James Clerk Maxwell
- Kinetic theory
References
- ↑ Synge, J.L., The relativistic gas, Noord-Holland, 1957
External links
- Maxwell Distribution at Mathworld
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
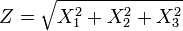
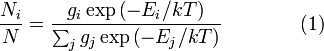
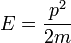
![\frac{N_i}{N} =
\frac{1}{Z}
\exp \left[
-\frac{p_x^2 + p_y^2 + p_z^2}{2mkT}
\right]
\qquad\qquad (3)](/2009-wikipedia_en_wp1-0.7_2009-05/I/8f527127e252ab832d3cae3e3ef3c2d3.png)
![f_\mathbf{p} (p_x, p_y, p_z) =
\frac{c}{Z}
\exp \left[
-\frac{p_x^2 + p_y^2 + p_z^2}{2mkT}
\right].
\qquad\qquad (4)](/2009-wikipedia_en_wp1-0.7_2009-05/I/abb62eba6fbe952980c6fa4d5f57618c.png)
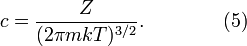
![f_\mathbf{p} (p_x, p_y, p_z) =
\left( \frac{1}{2 \pi mkT} \right)^{3/2}
\exp \left[
-\frac{p_x^2 + p_y^2 + p_z^2}{2mkT}
\right].
\qquad\qquad (6)](/2009-wikipedia_en_wp1-0.7_2009-05/I/c719a3700cd88ff5886b1d1d002a0ba6.png)
![f_E\,dE=f_p\left(\frac{dp}{dE}\right)\,dE =2\sqrt{\frac{E}{\pi(kT)^3}}~\exp\left[\frac{-E}{kT}\right]\,dE. \qquad \qquad(7)](/2009-wikipedia_en_wp1-0.7_2009-05/I/0bd1fa1f40fdbca865f37627c98a7518.png)
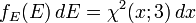
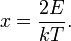
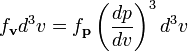
![f_\mathbf{v} (v_x, v_y, v_z) =
\left(\frac{m}{2 \pi kT} \right)^{3/2}
\exp \left[-
\frac{m(v_x^2 + v_y^2 + v_z^2)}{2kT}
\right],
\qquad\qquad](/2009-wikipedia_en_wp1-0.7_2009-05/I/85cf0ae8c15fbc5d914645b864430751.png)
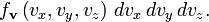
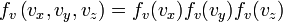
![f_v (v_i) =
\sqrt{\frac{m}{2 \pi kT}}
\exp \left[
\frac{-mv_i^2}{2kT}
\right].
\qquad\qquad](/2009-wikipedia_en_wp1-0.7_2009-05/I/295ead2949493bff9984603c3e07cd27.png)
![f (v) = 4 \pi
\left(\frac{m}{2 \pi kT}\right)^{3/2}\!\!v^2
\exp \left[
\frac{-mv^2}{2kT}
\right]
\qquad](/2009-wikipedia_en_wp1-0.7_2009-05/I/edcbd4b5f57cfeaedbfd1f086aa65e9d.png)