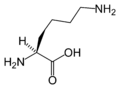
Lysine
| Lysine | |
|---|---|
 |
 |
| IUPAC name | 2,6-diaminohexanoic acid |
| Other names | 56-87-1 |
| Identifiers | |
| CAS number | |
| PubChem | |
| SMILES |
|
| ChemSpider ID | |
| Properties | |
| Molecular formula | C6H14N2O2 |
| Molar mass | 146.19 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references |
|
Lysine (abbreviated as Lys or K)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)(CH2)4NH2. This amino acid is an essential amino acid, which means that humans cannot synthesize it. Its codons are AAA and AAG.
Lysine is a base, as are arginine and histidine. The ε-amino group often participates in hydrogen bonding and as a general base in catalysis. Common posttranslational modifications include methylation of the ε-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications at lysine residues include acetylation and ubiquitination. Collagen contains hydroxylysine which is derived from lysine by lysyl hydroxylase. O-Glycosylation of lysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell.
Contents |
Biosynthesis
As an essential amino acid, lysine is not synthesized in animals, hence it must be ingested as lysine or lysine-containing proteins. In plants and microorganisms, it is synthesized from aspartic acid, which is first converted to β-aspartyl-semialdehyde. Cyclization gives dihydropicolinate, which is reduced to Δ1-piperidine-2,6-dicarboxylate. Ring-opening of this heterocycle gives a series of derivatives of pimelic acid, ultimately affording lysine. Enzymes involved in this biosynthesis include:[2]
- Aspartokinase
- β-aspartate semialdehyde dehydrogenase
- Dihydropicolinate synthase
- Δ1-piperidine-2,6-dicarboxylate dehydrogenase
- N-succinyl-2-amino-6ketopimelate synthase
- Succinyl diaminopimelate aminotransferase
- Succinyl diaminopimelate desuccinylase
- Diaminopimelate epimerase
- Diaminopimelate decarboxylase
Metabolism
Lysine is metabolised in mammals to give acetyl-CoA, via an initial transamination with α-ketoglutarate. The bacterial degradation of lysine yields cadaverine by decarboxylation.
Synthesis
Synthetic, racemic lysine has long been known.[3] A practical synthesis starts from caprolactam.[4]
Dietary sources
The human nutritional requirement is 1–1.5 g daily. It is the limiting amino acid (the essential amino acid found in the smallest quantity in the particular foodstuff) in all cereal grains, but is plentiful in all pulses (legumes). Plants that contain significant amounts of lysine include:
- Buffalo Gourd (10,130–33,000 ppm) in seed
- Berro, Watercress (1,340–26,800 ppm) in herb.
- Soybean (24,290–26,560 ppm) in seed.
- Carob, Locust Bean, St.John's-Bread (26,320 ppm) in seed;
- Common Bean (Black Bean, Dwarf Bean, Field Bean, Flageolet Bean, French Bean, Garden Bean, Green Bean, Haricot, Haricot Bean, Haricot Vert, Kidney Bean, Navy Bean, Pop Bean, Popping Bean, Snap Bean, String Bean, Wax Bean) (2,390–25,700 ppm) in sprout seedling;
- Ben Nut, Benzolive Tree, Jacinto (Sp.), Moringa (aka Drumstick Tree, Horseradish Tree, Ben Oil Tree), West Indian Ben (5,370–25,165 ppm) in shoot.
- Lentil (7,120–23,735 ppm) in sprout seedling.
- Asparagus Pea, Winged Bean (aka Goa Bean) (21,360–23,304 ppm) in seed.
- Fat Hen (3,540–22,550 ppm) in seed.
- Lentil (19,570–22,035 ppm) in seed.
- White Lupin (19,330–21,585 ppm) in seed.
- Black Caraway, Black Cumin, Fennel-Flower, Nutmeg-Flower, Roman Coriander (16,200–20,700 ppm) in seed.
- Spinach (1,740–20,664 ppm).
- Amaranth, Quinoa
- Kasha
Good sources of lysine are foods rich in protein including meat (specifically red meat, pork, and poultry), cheese (particularly Parmesan), certain fish (such as cod and sardines), and eggs.
Properties
L-Lysine is a necessary building block for all protein in the body. L-Lysine plays a major role in calcium absorption; building muscle protein; recovering from surgery or sports injuries; and the body's production of hormones, enzymes, and antibodies.
Modifications
Lysine can be modified through acetylation, methylation, ubiquitination, sumoylation, neddylation, biotinylation and carboxylation which tends to modify the function of the protein of which the modified lysine residue(s) are a part.[5]
Clinical significance
It has been suggested that lysine may be beneficial for those with herpes simplex infections.[6] However, more research is needed to fully substantiate this claim. For more information, refer to Herpes simplex - Lysine.
There are Lysine conjugates that show promise in the treatment of cancer, by causing cancerous cells to destroy themselves when the drug is combined with the use of phototherapy, while leaving non-cancerous cells unharmed.[7]
In popular culture
The 1993 film Jurassic Park, which is based on the 1989 Michael Crichton novel Jurassic Park, features dinosaurs that were genetically altered so they could not produce lysine.[8] This was supposed to prevent the cloned dinosaurs from leaving the park, forcing them to depend on lysine supplements provided by the park's veterinary staff. Most vertebrates cannot produce lysine by default (it is an essential amino acid).
The 2002 Max Tundra single "Lysine" (featuring Becky Jacobs) is a tribute to the advantages of lysine.[9]
The Indian children's vitamin tonic, Incremin, was claimed to supply this essential amino acid.
Linus Pauling believed people with existing atherosclerotic plaque can benefit from vitamin c and the amino acid lysine. Pauling filmed a video lecture in which he recommended that heart patients take between 2,000 and 6,000 mg of lysine daily with their vitamin C (more if serum Lp(a) is elevated).
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved on 2007-05-17.
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Braun, J. V. “Synthese des inaktiven Lysins aus Piperidin" Berichte der deutschen chemischen Gesellschaft 1909, Volume 42, p 839-846. DOI: 10.1002/cber.190904201134.
- ↑ Eck, J. C.; Marvel, C. S. “dl-Lysine Hydrochlorides” Organic Syntheses, Collected Volume 2, p.374 (1943). http://www.orgsyn.org/orgsyn/pdfs/CV2P0374.pdf
- ↑ Sadoul K, Boyault C, Pabion M, Khochbin S (February 2008). "Regulation of protein turnover by acetyltransferases and deacetylases". Biochimie 90 (2): 306–12. doi:. PMID 17681659. http://linkinghub.elsevier.com/retrieve/pii/S0300-9084(07)00168-X.
- ↑ Griffith RS, Norins AL, Kagan C. (1978). "A multicentered study of lysine therapy in Herpes simplex infection". Dermatologica. 156 (5): 257–267. PMID 640102.
- ↑ ScienceDaily. "Chemists Kill Cancer Cells With Light-activated Molecules". Retrieved on 2008-01-24.
- ↑ Coyne, Jerry A. (October 10 1999). "The Truth Is Way Out There", The New York Times. Retrieved on 2008-04-06.
- ↑ "Lysine (CDep)". Insound. Retrieved on 2008-04-06.
See also
- Acetyllysine
- Lysine price-fixing conspiracy
- Deamination
- Saccharopine
Sources
- Much of the information in this article has been translated from German Wikipedia.
- Lide, D. R., ed. (2002), CRC Handbook of Chemistry and Physics (83rd ed.), Boca Raton, FL: CRC Press, ISBN 0-8493-0483-0
External links
|
||||||||||||||