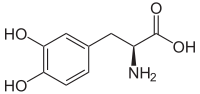
L-DOPA
 |
|
|
L-DOPA
|
|
| Systematic (IUPAC) name | |
| (S)-2-amino-3-(3,4-dihydroxyphenyl) propanoic acid |
|
| Identifiers | |
| CAS number | |
| ATC code | N04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | C9H11NO4 |
| Mol. mass | 197.19 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Metabolism | Aromatic-L-amino-acid decarboxylase |
| Half life | 0.75–1.5 hours |
| Excretion | renal 70–80% |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
OTC |
| Routes | oral |
L-DOPA (3,4-dihydroxy-L-phenylalanine) is a naturally occurring amino acid found in food and made from L-Tyrosine in the human body. L-DOPA is converted into dopamine in the brain and body. It is sold as a dietary supplement and as a prescription drug in the US. In clinical use, Levodopa (INN) is administered in the management of Parkinson's disease and Dopa-Responsive Dystonia. It is also used as a component in marine adhesives used by pelagic life.
Contents |
Therapeutic use
L-Dopa is used to increase dopamine levels for the treatment of Parkinson's disease and Dopa-Responsive Dystonia, since it is able to cross the blood-brain barrier, whereas dopamine itself cannot. Once levodopa has entered the central nervous system (CNS), it is metabolized to dopamine by aromatic L-amino acid decarboxylase. Pyridoxal phosphate (vitamin B6) is a required cofactor for this decarboxylation, and may be administered along with levodopa, usually as pyridoxine.
Conversion to dopamine also occurs in the peripheral tissues, i.e. outside the brain. This may be the mechanism of the adverse effects of levodopa. It is standard clinical practice to co-administer a peripheral DOPA decarboxylase inhibitor—carbidopa or benserazide—and often a catechol-O-methyl transferase (COMT) inhibitor, to prevent synthesis of dopamine in peripheral tissue. Co-administration of pyridoxine without a decarboxylase inhibitor accelerates the extracerebral decarboxylation to such an extent that it cancels out the effects of levodopa administration, a circumstance which historically caused great confusion.
For those taking it as a supplement, EGCG or green tea is a natural decarboxylase inhibitor.
Levodopa, co-administered with a peripheral DOPA decarboxylase inhibitor, has been tested as a possible treatment for restless leg syndrome (RLS) and shown "no clear picture of reduced symptoms".[1]
Adverse effects
Possible adverse drug reactions include:
- Hypotension, especially if the dosage is too high
- Arrhythmias, although these are uncommon
- Nausea, which is often reduced by taking the drug with food, although protein interferes with drug absorption
- Gastrointestinal bleeding
- Disturbed respiration, which is not always harmful, and can actually benefit patients with upper airway obstruction
- Hair loss
- Confusion
- Extreme emotional states, particularly anxiety, but also excessive libido
- Vivid dreams and/or fragmented sleep
- Visual and possibly auditory hallucinations
- Effects on learning; there is some evidence that it improves working memory, while impairing other complex functions
- Sleepiness and sleep attacks
- A condition similar to amphetamine psychosis.
Although there are many adverse effects associated with levodopa, particularly psychiatric ones, it has fewer than other anti-Parkinson's drugs, including anticholinergics, amantadine, and dopamine agonists.
More serious are the effects of chronic levodopa administration, which include:
- End-of-dose deterioration of function
- On/off oscillations
- Freezing during movement
- Dose failure (drug resistance)
- Dyskinesia at peak dose.
- Recent studies have demonstrated that use of L-dopa without simultaneously giving proper levels of serotonin percursors depletes serotonin.
Clinicians will try to avoid these by limiting levodopa dosages as far as possible until absolutely necessary.
Toxicity
Some studies suggest a cytotoxic role in the promotion and occurrence of adverse effects associated with levodopa treatment.[2] Though the drug is generally safe in humans, some researchers have reported an increase in cytotoxicity markers in rat pheochromocytoma PC12 cell lines treated with levodopa.[3] Other authors have attributed the observed toxic effects of levodopa in neural dopamine cell lines to enhanced formation of quinones through increased auto-oxidation and subsequent cell death in mesencephalic cell cultures.[4][5] Though levodopa is generally considered safe, some controversy surrounds use of the drug in Parkinson's Disease given some data indicating a deleterious effect on intracellular and neuronal tissue involved in the pathogenesis of the disease.[6]
Biosynthesis

L-DOPA is produced from the amino acid tyrosine by the enzyme tyrosine hydroxylase. It is also the precursor molecule for the catecholamine neurotransmitters dopamine and norepinephrine (noradrenaline), and the hormone epinephrine (adrenaline). Dopamine is formed by the decarboxylation of L-DOPA.
L-DOPA can be directly metabolized by catechol-O-methyl transferase (COMT) to 3-O-methyldopa (3-OMD) and then further to vanillactic acid (VLA). This metabolic pathway is non-existent in the healthy body but becomes important after peripheral L-DOPA administration in patients with Parkinson's Disease or in the rare cases of patients with aromatic L-amino acid decarboxylase (AADC) enzyme deficiency.[7]
The prefix L- references its property of levorotation (compared with dextrorotation or D-DOPA).
History
In work that earned him a Nobel Prize in 2000, Swedish scientist Arvid Carlsson first showed in the 1950s that administering levodopa to animals with Parkinsonian symptoms would cause a reduction of the symptoms. The neurologist Oliver Sacks describes this treatment in human patients with encephalitis lethargica in his book Awakenings, upon which the movie of the same name is based.
The 2001 Nobel Prize in Chemistry was also related to L-DOPA: the Nobel Committee awarded one-fourth of the prize to William S. Knowles for his work on chirally-catalysed hydrogenation reactions, the most noted example of which was used for the synthesis of L-DOPA.
Supplements containing L-DOPA
Herbal supplements containing standardized dosages of L-DOPA are available without a prescription. These supplements have recently increased in both availability and popularity in the United States and on the internet. The most common plant source of L-DOPA marketed in this manner is a tropical legume, Mucuna pruriens, also known as "Velvet Bean" and by a number of other common names.
Adhesion
DOPA is a key molecule in the formation of marine adhesive proteins, such as those found in mussels. It is believed to be responsible for the water-resistance and rapid curing abilities of these proteins. DOPA may also be used to prevent surfaces from fouling by bonding antifouling polymers to a susceptible substrate.
Melanin formation
Both levodopa and its precursor amino acid L-tyrosine are precursors to the biological pigment melanin. The enzyme tyrosinase catalyzes the oxidation of L-dopa to the reactive intermediate dopaquinone, which reacts further, eventually leading to melanin oligomers.
Footnotes
- ↑ "L-dopa for RLS". Bandolier (1 April 2007). Retrieved on 2008-10-16.
- ↑ Cheng N, Maeda T, Kume T, et al (December 1996). "Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons". Brain research 743 (1-2): 278–83. PMID 9017256.
- ↑ Basma AN, Morris EJ, Nicklas WJ, Geller HM (February 1995). "L-dopa cytotoxicity to PC12 cells in culture is via its autoxidation". Journal of neurochemistry 64 (2): 825–32. PMID 7830076.
- ↑ Pardo B, Mena MA, Casarejos MJ, Paíno CL, De Yébenes JG (June 1995). "Toxic effects of L-DOPA on mesencephalic cell cultures: protection with antioxidants". Brain research 682 (1-2): 133–43. PMID 7552304.
- ↑ Mytilineou C, Han SK, Cohen G (October 1993). "Toxic and protective effects of L-dopa on mesencephalic cell cultures". Journal of neurochemistry 61 (4): 1470–8. PMID 8376999.
- ↑ Simuni T, Stern MB (June 1999). "Does levodopa accelerate Parkinson's disease?". Drugs & aging 14 (6): 399–408. PMID 10408739.
- ↑ Hyland K, Clayton PT (December 1992). "Aromatic L-amino acid decarboxylase deficiency: diagnostic methodology" (PDF). Clinical chemistry 38 (12): 2405–10. PMID 1281049. http://www.clinchem.org/cgi/reprint/38/12/2405.pdf.
References
- Waite, J. Herbert, et al. (2005). "Mussel Adhesion: Finding the Tricks Worth Mimicking". J Adhesion 81: 1–21. doi:.
- Messersmith, Phillip B., et al. (2006). "Rapid Gel Formation and Adhesion in Photocurable and Biodegradable Block Copolymers with High DOPA Content". Macromolecules 39: 1740–1748. doi:.
External links
- ScienceDaily: Study Reveals Details Of Mussels' Tenacious Bonds (Northwestern University) August 16, 2006
|
|||||||||||||||||||||||||
|
|||||||||||||||||