Krypton
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | krypton, Kr, 36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | noble gases | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 18, 4, p | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | colorless gas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 83.798(2) g·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 4p6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | gas | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | (0 °C, 101.325 kPa) 3.749 g/L |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 115.79 K (-157.36 °C, -251.25 °F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 119.93 K (-153.22 °C, -244.12 °F) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 115.775 K, 73.2 kPa[1] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 209.41 K, 5.50 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 1.64 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 9.08 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | (25 °C) 20.786 J·mol−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic face centered | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4,[2] 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 3.00 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies (more) |
1st: 1350.8 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd: 2350.4 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3rd: 3565 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calc.) | 88 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 110 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 202 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | nonmagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 9.43x10-3 W·m−1·K−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | (gas, 23 °C) 220 m/s | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | (liquid) 1120 m/s | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7439-90-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most-stable isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Krypton (pronounced /ˈkrɪptən/ or /ˈkrɪptɒn/; from Greek: kryptos "hidden") is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other rare gases in fluorescent lamps. Krypton is inert for most practical purposes. Krypton can also form clathrates with water when atoms of it are trapped in a lattice of the water molecules.
Krypton, like the other noble gases, can be used in lighting and photography. Krypton light has a large number of spectral lines, and krypton's high light output in plasmas allows it to play an important role in many high-powered gas lasers, which pick out one of the many spectral lines to amplify. There is also a specific krypton fluoride laser. The high power and relative ease of operation of krypton discharge tubes caused (from 1960 to 1983), the official metre (metric distance) to be defined in terms of one orange-red spectral line of krypton-86.
Contents |
Physical properties

Krypton is characterized by a brilliant green and orange spectral signature. It is one of the products of uranium fission.[3] Solidified krypton is white and crystalline with a face-centered cubic crystal structure, which is a common property of all noble gases.
History
Krypton was discovered in Britain in 1898 by Sir William Ramsay a Scottish chemist and Morris Travers an English chemist in residue left from evaporating nearly all components of liquid air.[4] William Ramsay was awarded the 1904 Nobel Prize in Chemistry for discovery of a series of noble gases, including krypton.
Metric role
In 1960, an international agreement defined the metre in terms of wavelength of light emitted by the krypton-86 isotope. This agreement replaced the longstanding standard metre located in Paris, which was a metal bar made of a platinum-iridium alloy (the bar was originally estimated to be one ten-millionth of a quadrant of the earth's polar circumference), and was itself replaced by a definition based on the speed of light — a fundamental physical constant. In October 1983, the Bureau International des Poids et Mesures (International Bureau of Weights and Measures) defined the meter as the distance that light travels in a vacuum during 1/299,792,458 s.[5]
Occurrence
The Earth has retained all of the noble gases that were present at its formation except for helium. Helium atoms are very light, and move fast enough to escape the earth's gravity readily.[6] Krypton's concentration in the atmosphere is about 1 ppm. It can be extracted from liquid air by fractional distillation.[7] The amount of krypton in space is uncertain, as is the amount is derived from the meteoritic activity and that from solar winds. The first measurements suggest an overabundance of krypton in space.[8]
Compounds
Like the other noble gases, krypton is chemically unreactive. However, following the first successful synthesis of xenon compounds in 1962, synthesis of krypton difluoride was reported in 1963.[9] There are unverified reports of other fluorides and a salt of a krypton oxoacid. ArKr+ and KrH+ molecule-ions have been investigated and there is evidence for KrXe or KrXe+.[10]
At the University of Helsinki in Finland, HKrCN and HKrCCH (krypton hydride-cyanide and hydrokryptoacetylene) were synthesized and determined to be stable up to 40 K.[9]
Isotopes
There are 20 known isotopes of krypton.[11] Naturally occurring krypton is made of five stable and one slightly radioactive isotope. Its spectral signature can be produced with some very sharp lines. 81Kr, the product of atmospheric reactions is produced with the other naturally occurring isotopes of krypton. Being radioactive it has a half-life of 230,000 years. Krypton is highly volatile when it is near surface waters but 81Kr has been used for dating old (50,000 - 800,000 year) groundwater.[12]
85Kr is an inert radioactive noble gas with a half-life of 10.76 years. It is produced by the fission of uranium and plutonium, such as in nuclear bomb testing and nuclear reactors. 85Kr is released during the reprocessing of fuel rods from nuclear reactors. Concentrations at the North Pole are 30% higher than at the South Pole as most nuclear reactors are in the northern hemisphere.[13]
Applications
Krypton's multiple emission lines make ionized krypton gas discharges appear whitish, which in turn makes krypton-based bulbs useful in photography as a brilliant white light source. Krypton is thus used in some types of photographic flashes used in high speed photography. Krypton gas is also combined with other gases to make luminous signs that glow with a bright greenish-yellow light.[14]
Krypton is mixed with argon as the fill gas of energy saving fluorescent lamps. This reduces their operating voltage and power consumption. Unfortunately it also reduces their light output and raises their cost.[15] Krypton costs 100 times as much as argon. Krypton (along with xenon) is also used to fill incandescent lamps to reduce filament evaporation and allow higher operating temperatures to be used for the filament.[16] A brighter light results which contains more blue than conventional lamps.
Krypton's white discharge is often used to good effect in coloured gas discharge tubes, which are then simply painted or stained in other ways to allow the desired colour (for example, "neon" type advertising signs where the letters appear in differing colours, are often entirely krypton-based). Krypton is also capable of much higher light power density than neon in the red spectral line region, and for this reason, red lasers for high power laser light shows are often krypton lasers with mirrors which select out the red spectral line for laser amplification and emission, rather than the more familiar helium-neon variety, which could never practically achieve the multi-watt red laser light outputs needed for this application.[17]
Krypton has an important role in production and usage of the krypton fluoride laser. The laser has been important in the nuclear fusion energy research community in confinement experiments. The laser has high beam uniformity, short wavelength, and the ability to modify the spot size to track an imploding pellet.[18]
In experimental particle physics, liquid krypton is used to construct quasi-homogeneous electromagnetic calorimeters. A notable example is the calorimeter of the NA48 experiment at CERN containing about 27 tons of liquid krypton. This usage is rare, since the cheaper liquid argon is typically used. The advantage of krypton over argon is a small Molière radius of 4.7cm, which allows for excellent spatial resolution and low degree of overlapping. The other parameters relevant for calorimetry application are: radiation length of 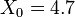 cm, density of 2.4g/cm³.
cm, density of 2.4g/cm³.
The sealed spark gap assemblies contained in ignition excitors used in some older Turbine/Jet engines contain a very small amount of Krypton 85 in order to obtain consistent ionization levels and uniform operation. The amount of radiation from the average gap is approximately the same as that of a radium-dialed wrist watch but should be handled carefully.
References
- ↑ "Section 4, Properties of the Elements and Inorganic Compounds; Melting, boiling, triple, and critical temperatures of the elements". CRC Handbook of Chemistry and Physics (85th edition ed.). Boca Raton, Florida: CRC Press. 2005.
- ↑ "Krypton: krypton(IV) fluoride compound data". Books.Google.com. Retrieved on 2007-12-10.
- ↑ "Krypton" (in English) 1. Argonne National Laboratory, EVS (08 2005). Retrieved on 2007-03-17.
- ↑ William Ramsay, Morris W. Travers (1898). "On a New Constituent of Atmospheric Air". Proceedings of the Royal Society of London 63: 405–408. doi:. http://links.jstor.org/sici?sici=0370-1662%281898%2963%3C405%3AOANCOA%3E2.0.CO%3B2-M.
- ↑ Gibbs, Philip (1997). "How is the speed of light measured?" (in English). Department of Mathematics, University of California. Retrieved on 2007-03-19.
- ↑ Escape of Gases from the Atmosphere
- ↑ "How Products are Made: Krypton". Retrieved on 2006-07-02.
- ↑ Cardelli, Jason A.; Meyer, David M. (18). "The Abundance of Interstellar Krypton" (in English). The Astrophysical Journal Letters L57–L60. The American Astronomical Society. Retrieved on 2007-04-05.
- ↑ 9.0 9.1 Bartlett, Neil (2003). "The Noble Gases" (in English). Chemical & Engineering News. Retrieved on 2006-07-02.
- ↑ "Periodic Table of the Elements" (in English) 100-101. Los Alamos National Laboratory's Chemistry Division. Retrieved on 2007-04-05.
- ↑ "Isotopes of Krypton". Nuclear Science Division. Retrieved on 2007-03-20.
- ↑ Thonnard, Norbert; Larry D. MeKay, Theodore C. Labotka (31). "Development of Laser-Based Resonance Ionization Techniques for 81-Kr and 85-Kr Measurements in the Geosciences" (in English) 4-7. University of Tennessee, Institute for Rare Isotope Measurements. Retrieved on 2007-03-20.
- ↑ "Resources on Isotopes". U.S. Geological Survey. Retrieved on 2007-03-20.
- ↑ "Mercury in Lighting". Cape Cod Cooperative Extension. Retrieved on 2007-03-20.
- ↑ "Energy-saving" lamps
- ↑ Properties, Applications and Uses of the "Rare Gases" Neon, Krypton and Xenon
- ↑ "Laser Devices, Laser Shows and Effect" (PDF). Retrieved on 2007-04-05.
- ↑ Sethian, J.; M. Friedman, M.Myers. "Krypton Fluoride Laser Development for Inertial Fusion Energy" (in English) 1-8. Plasma Physics Division, Naval Research Laboratory. Retrieved on 2007-03-20.
Further reading
- Los Alamos National Laboratory - Krypton
- "Chemical Elements: From Carbon to Krypton" By: David Newton & Lawrence W. Baker
- "Krypton 85: a Review of the Literature and an Analysis of Radiation Hazards" By: William P. Kirk.
External links
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||