Kidney stone
| Kidney stone Classification and external resources |
|
 |
|
|---|---|
| An 8 mm kidney stone. | |
| ICD-10 | N20.0 |
| ICD-9 | 592.0 |
| DiseasesDB | 11346 |
| MedlinePlus | 000458 |
| eMedicine | med/1600 |
| MeSH | D007669 |
Kidney stones, also called renal calculi, are solid concretions (crystal aggregations) of dissolved minerals in urine; calculi typically form inside the kidneys or bladder. The terms nephrolithiasis and urolithiasis refer to the presence of calculi in the kidneys and urinary tract, respectively.
Contents |
Overview
The kidneys are a pair of organs that are primarily responsible for filtering metabolites and minerals from the circulatory system. These secretions are then passed to the bladder and out of the body as urine. Some of the substances found in urine are able to crystalize, and in a concentrated form these chemicals can precipitate into a solid deposit attached to the kidney walls. These crystals can grow through a process of accretion to form a kidney stone.[1] In medical terminology these deposits are known as renal calculi (Latin renal, "kidney" and calculi, "pebbles").[2]
Renal calculi can vary in size from as small as grains of sand to as large as a golf ball.[3] Kidney stones typically leave the body by passage in the urine stream, and many stones are formed and passed without causing symptoms. If stones grow to sufficient size before passage—on the order of at least 2-3 millimeters—they can cause obstruction of the ureter. The resulting obstruction with dilation or stretching of the upper ureter and renal pelvis as well as spasm of muscle, trying to move the stone, can cause severe episodic pain, most commonly felt in the flank, lower abdomen and groin (a condition called renal colic). Renal colic can be associated with nausea and vomiting due to the embryological association of the kidneys with the intestinal tract. Hematuria (bloody urine) is commonly present due to damage to the lining of the urinary tract.
Within the United States, about 10–15% of adults will be diagnosed with a kidney stone,[4] and the total cost for treating this condition was US$2 billion in 2003.[5] The incidence rate increases to 20–25% in the Middle East, because of increased risk of dehydration in hot climates. (The typical Arabian diet is also 50% lower in calcium and 250% higher in oxalates compared to Western diets, increasing the net risk.)[6] Recurrence rates are estimated at about 10% per year, totalling 50% over a 5–10 year period and 75% over 20 years.[7] Men are affected approximately 4 times more often than women. Recent evidence has shown an increase in pediatric cases.[8]
History
The existence of kidney stones has been recorded since the beginning of civilization, and lithotomy for the removal of stones is one of the earliest known surgical procedures.[9] In 1901, a stone was discovered in the pelvis of an ancient Egyptian mummy, and was dated to 4,800 BCE. Medical text from ancient Mesopotamia, India, China, Persia, Greece and Rome all mentioned calculous disease. Part of the Hippocratic oath contains an admonition about the dangers of operating on the bladder for stones. The Roman medical treatise De Medicina by Cornelius Celsus contained a description of lithotomy, and this work served as the basis for this procedure up until the 18th century.[10]
New techniques in lithotomy began to emerge starting in 1520, but the operation remained risky. It was only after Henry J. Bigelow popularized the technique of litholopaxy in 1878 that the mortality rate dropped from about 24% down to 2.4%. However, other treatment techniques were developed that continued to produce a high level of mortality, especially among inexperienced urologists.[10][11] In 1980, Dornier MedTech introduced extracorporeal shock wave lithotripsy for breaking up stones via acoustical pulses, and this technique has come into widespread use.[12]
Among the famous leaders who were kidney stone formers are Emperor Napoleon Bonaparte, Emperor Napoleon III, Peter the Great, Louis XIV, George IV, Oliver Cromwell, and former U.S. President Lyndon B. Johnson. Other notable individuals who endured stones include Benjamin Franklin, the philosophers Michel de Montaigne and Sir Francis Bacon, the scientist Sir Isaac Newton, the civil servant and diarist Samuel Pepys, the physicians William Harvey and Herman Boerhaave, and the anatomist Antonio Scarpa.[11] Astronauts seem to show a higher risk of developing kidney stones during or after long duration space flights.[13]
Causes

Kidney stones can be due to underlying metabolic conditions, such as renal tubular acidosis,[7] Dent's disease[14] and medullary sponge kidney.[15] Many health facilities will screen for such disorders in patients with recurrent kidney stones. This is typically done with a 24 hour urine collection that is chemically analyzed for deficiencies and excesses that promote stone formation. Kidney stones are also more common in patients with Crohn's disease.[16]
There has been some evidence that water fluoridation may increase the risk of kidney stone formation. In one study, patients with symptoms of skeletal fluorosis were 4.6 times as likely to develop kidney stones.[17] However, fluoride may also be an inhibitor of urinary stone formation.[18]
There is a longstanding belief among the mainstream medical community that vitamin C causes kidney stones, which may be based on little science.[19] Although some individual recent studies have found a relationship[20] there is no clear relationship between excess ascorbic acid intake and kidney stone formation. [21]
Calcium oxalate stones
The most common type of kidney stone is composed of calcium oxalate crystals, occurring in about 80% of cases,[7] and the factors that promote the precipitation of crystals in the urine are associated with the development of these stones.
Common sense has long held that consumption of too much calcium could promote the development of calcium kidney stones. However, current evidence suggests that the consumption of low-calcium diets is actually associated with a higher overall risk for the development of kidney stones.[22] This is perhaps related to the role of calcium in binding ingested oxalate in the gastrointestinal tract. As the amount of calcium intake decreases, the amount of oxalate available for absorption into the bloodstream increases; this oxalate is then excreted in greater amounts into the urine by the kidneys. In the urine, oxalate is a very strong promoter of calcium oxalate precipitation, about 15 times stronger than calcium.
Uric acid (urate)
About 5–10% of all stones are formed from uric acid.[7] Uric acid stones form in association with conditions that cause hyperuricosuria with or without high blood serum uric acid levels (hyperuricemia); and with acid/base metabolism disorders where the urine is excessively acidic (low pH) resulting in uric acid precipitation. A diagnosis of uric acid nephrolithiasis is supported if there is a radiolucent stone, a persistent undue urine acidity, and uric acid crystals in fresh urine samples.[23]
Other types
Other types of kidney stones are composed of struvite (magnesium, ammonium and phosphate); calcium phosphate; and cystine.
The formation of struvite stones is associated with the presence of urea-splitting bacteria, most commonly Proteus mirabilis (but also Klebsiella, Serratia, Providencia species). These organisms are capable of splitting urea into ammonia, decreasing the acidity of the urine and resulting in favorable conditions for the formation of struvite stones. Struvite stones are always associated with urinary tract infections.
The formation of calcium phosphate stones is associated with conditions such as hyperparathyroidism and renal tubular acidosis.
Formation of cystine stones is uniquely associated with people suffering from cystinuria, who accumulate cystine in their urine.
Urolithiasis has also been noted to occur in the setting of therapeutic drug use, with crystals of drug forming within the renal tract in some patients currently being treated with Indinavir, Sulfadiazine or Triamterene [24].
Symptoms
Symptoms of kidney stones include:[22][3]
- Colicky pain: "loin to groin". Often described as "the worst pain [...] ever experienced".[25]
- Hematuria: blood in the urine, due to minor damage to inside wall of kidney, ureter and/or urethra.
- Pyuria: pus in the urine.
- Dysuria: burning on urination when passing stones (rare). More typical of infection.
- Oliguria: reduced urinary volume caused by obstruction of the bladder or urethra by stone, or extremely rarely, simultaneous obstruction of both ureters by a stone.
- Abdominal distention.
- Nausea/vomiting: embryological link with intestine – stimulates the vomiting center.
- Fever and chills.
- Hydronephrosis[26]
- Postrenal azotemia: when kidney stone blocks ureter[27]
Diagnosis

Clinical diagnosis is usually made on the basis of the location and severity of the pain, which is typically colic in nature (comes and goes in spasmodic waves). Pain in the back occurs when calculi produce an obstruction in the kidney.[3]
Imaging is used to confirm the diagnosis and a number of other tests can be undertaken to help establish both the possible cause and consequences of the stone. Ultrasound imaging is also useful as it will give details about the presence of hydronephrosis (swelling of the kidney—suggesting the stone is blocking the outflow of urine).[5] It can also be used to show the kidneys during pregnancy when standard x-rays are discouraged. About 10% of stones do not have enough calcium to be seen on standard x-rays (radiolucent stones) and may show up on ultrasound although they typically are seen on CT scans.
The relatively dense calcium renders these stones radio-opaque and they can be detected by a traditional X-ray of the abdomen that includes the Kidneys, Ureters and Bladder—KUB.[5] This may be followed by an IVP (Intravenous Pyelogram; (IntraVenous Urogram (IVU) is the same test by another name)) which requires about 50 ml of a special dye to be injected into the bloodstream that is excreted by the kidneys and by its density helps outline any stone on a repeated X-ray. These can also be detected by a Retrograde pyelogram where similar "dye" is injected directly into the ureteral opening in the bladder by a surgeon, usually a urologist.
Computed tomography (CT or CAT scan), a specialized X-ray, is considered the gold-standard diagnostic test for the detection of kidney stones, and in this setting does not require the use of intravenous contrast, which carries some risk in certain people (eg, allergy, kidney damage). All stones are detectable by CT except very rare stones composed of certain drug residues in the urine.[5] The non-contrast "renal colic study" CT scan has become the standard test for the immediate diagnosis of flank pain typical of a kidney stone. If positive for stones, a single standard x-ray of the abdomen (KUB) is recommended. This additional x-ray provides the physicians with a clearer idea of the exact size and shape of the stone as well as its surgical orientation. Further, it makes it simple to follow the progress of the stone without the need for the much more expensive CT scan just by doing another single x-ray at some point in the future.
Other investigations typically carried out include:[5]
- Microscopic study of urine, which may show proteins, red blood cells, bacteria, cellular casts and crystals.
- Culture of a urine sample to exclude urine infection (either as a differential cause of the patient's pain, or secondary to the presence of a stone)
- Blood tests: Full blood count for the presence of a raised white cell count (Neutrophilia) suggestive of infection, a check of renal function and to look for abnormally high blood calcium blood levels (hypercalcaemia).
- 24 hour urine collection to measure total daily urinary volume, magnesium, sodium, uric acid, calcium, citrate, oxalate and phosphate.
- Catching of passed stones at home (usually by urinating through a tea strainer) for later examination and evaluation by a doctor.[28][29]
Treatment
Temporizing
About 90% of stones 4 mm or less in size usually will pass spontaneously, however 99% of stones larger than 6 mm will require some form of intervention.[30] There are various measures that can be used to encourage the passage of a stone. These can include increased hydration, medication for treating infection and reducing pain, and diuretics to encourage urine flow and prevent further stone formation. Eating starfruit can be effective at reducing pain and improving urination.[3] However caution should be exercised due to other concerns with the ingestion of starfruit.[31]
In most cases, a smaller stone that is not symptomatic is often given up to four weeks[22] to move or pass before consideration is given to any surgical intervention as it has been found that waiting longer tends to lead to additional complications. Immediate surgery may be required in certain situations such as in people with only one working kidney, intractable pain or in the presence of an infected kidney blocked by a stone which can rapidly cause severe sepsis and toxic shock.
Analgesia
Management of pain from kidney stones varies from country to country and even from physician to physician, but usually requires intravenous administration of narcotics in an emergency room setting for acute situations. Similar classes of drugs may be reasonably effective orally in an outpatient setting for less severe discomfort where nonsteroidal anti-inflammatories or narcotics like codeine can be prescribed. Some doctors will give patients with recurring passing of small stones a small supply prescription for hydrocodone to avoid a future visit to the ER when the next episode occurs. Taken at the first sign of pain, hydrocodone can eliminate much of the acute pain, nausea and vomiting which necessitates the hospital visit and still facilitate stone passage, although a follow-up with a physician is still necessary.
Patients who are to be treated non-surgically, may also be started on an alpha adrenergic blocking agent (such as Flomax, Uroxatral, terazosin or doxazosin), which acts to reduce the muscle tone of the ureter and facilitate stone passage. For smaller stones near the bladder, this type of medical treatment can increase the spontaneous stone passage rate by about 30%.
After treatment, the pain may return if the stone moves but re-obstructs in another location. Patients are encouraged to strain their urine so they can collect the stone when it eventually passes and send it for chemical composition analysis which will be used along with a 24 hour urine chemical analysis test to establish preventative options.
Urologic interventions


Most kidney stones do not require surgery and will pass on their own. Surgery is necessary when the pain is persistent and severe, in renal failure and when there is a kidney infection. It may also be advisable if the stone fails to pass or move after 30 days. Finding a significant stone before it passes into the ureter allows physicians to fragment it surgically before it causes any severe problems. In most of these cases, non-invasive Extracorporeal Shock Wave Lithotripsy (ESWL) will be used. Otherwise some form of invasive procedure is required; with approaches including ureteroscopic fragmentation (or simple basket extraction if feasible) using laser, ultrasonic or mechanical (pneumatic, shock-wave) forms of energy to fragment the larger stones. Percutaneous nephrolithotomy or rarely open surgery may ultimately be necessary for large or complicated stones or stones which fail other less invasive attempts at treatment.
A single retrospective study in the USA, at the Mayo Clinic, has suggested that lithotripsy may increase subsequent incidence of diabetes and hypertension,[32] but it has not been felt warranted to change clinical practice at the clinic.[33] The study reflects early experience with the original lithotripsy machine which had a very large blast path, much larger than what is used on modern machines. Further study is believed necessary to determine how much risk this treatment actually has using modern machines and treatment regimens.
More common complications related to ESWL are bleeding, pain related to passage of stone fragments, failure to fragment the stone, and the possible requirement for additional or alternative interventions.
Ureteral (double-J) stents
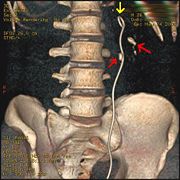
One modern medical technique uses a ureteral stent (a small tube between the bladder and the inside of the kidney) to provide immediate relief of a blocked kidney. This is especially useful in saving a failing kidney due to swelling and infection from the stone. Ureteral stents vary in length and width but most have the same shape usually called a "double-J" or "double pigtail", because of the curl at both ends. They are designed to allow urine to drain around any stone or obstruction. They can be retained for some length of time as infections recede and as stones are dissolved or fragmented with ESWL or other treatment. The stents will gently dilate or stretch the ureters which can facilitate instrumentation and they will also provide a clear landmark to help surgeons see the stones on x-ray. Most stents can be removed easily during a final office visit. Discomfort levels from stents typically range from minimal associated pain to moderate discomfort.
Prevention
Preventive strategies include dietary modifications and sometimes also taking drugs with the goal of reducing excretory load on the kidneys:[34][22]
- Drinking enough water to make 2 to 2.5 liters of urine per day.
- A diet low in protein, nitrogen and sodium intake.
- Restriction of oxalate-rich foods, such as chocolate, nuts, soybeans,[35] rhubarb and spinach, plus maintenance of an adequate intake of dietary calcium. There is equivocal evidence that calcium supplements increase the risk of stone formation, though calcium citrate appears to carry the lowest, if any, risk.
- Taking drugs such as thiazides, potassium citrate, magnesium citrate and allopurinol, depending on the cause of stone formation.
- Some fruit juices, such as orange, blackcurrant, and cranberry, may be useful for lowering the risk factors for specific types of stones.[36][37]
- Avoidance of cola beverages.[38][39]
- Avoiding large doses of vitamin C.[40]
For those patients interested in optimizing their kidney stone prevention options, it's essential to have a 24 hour urine test performed. This should be done with the patient on his or her regular diet and activities. The results can then be analyzed for abnormalities and appropriate treatment given.
Restricting Oxalate consumption
Calcium plays a vital role in body chemistry so limiting Calcium is unhealthy. Since Calcium in the intestinal tract will bind with available Oxalate, thereby preventing its absorption into the blood stream, some Nephrologists recommend chewing Calcium tablets during meals containing Oxalate foods. However, a more reliable approach is to restrict the intake of food that is high in Oxalate.
| Common high-Oxalate foods[41] | ||
|---|---|---|
| Food Item | Serving (oz.) |
Oxalate Content (mg) |
| Beet greens, cooked | 1/2 cup | 916 |
| Purslane, leaves, cooked | 1/2 cup | 910 |
| Rhubarb, stewed, no sugar | 1/2 cup | 860 |
| Spinach, cooked | 1/2 cup | 750 |
| Beets, cooked | 1/2 cup | 675 |
| Chard, Swiss, leaves cooked | 1/2 cup | 660 |
| Rhubarb, canned | 1/2 cup | 600 |
| Spinach, frozen | 1/2 cup | 600 |
| Beets, pickled | 1/2 cup | 500 |
| Poke Greens, cooked | 1/2 cup | 476 |
| Endive, raw | 20 long leaves | 273 |
| Cocoa, dry | 1/3 cup | 254 |
| Dandelion Greens, cooked | 1/2 cup | 246 |
| Okra, cooked | 8 - 9 pods | 146 |
| Potatoes, sweet, cooked | 1/2 cup | 141 |
| Kale, cooked | 1/2 cup | 125 |
| Peanuts, raw | 1/3 cup (1-3/4 oz) | 113 |
| Turnip Greens, cooked | 1/2 cup | 110 |
| Chocolate, unsweetened | 1 oz. | 91 |
| Parsnips, diced, cooked | 1/2 cup | 81 |
| Collard Greens, cooked | 1/2 cup | 74 |
| Pecans, halves, raw | 1/3 cup (1-1/4 oz) | 74 |
| Tea, leaves ( 4 min. infusion) | 1 level tsp in 7 oz water | 72 |
| Wheat Germ, toasted | 1/4 cup | 67 |
| Gooseberries | 1/2 cup | 66 |
| Potato, Idaho white, baked | 1 medium | 64 |
| Carrots, cooked | 1/2 cup | 45 |
| Apple, raw with skin | 1 medium | 41 |
| Brussel sprouts, cooked | 6 - 8 medium | 37 |
| Strawberries, raw | 1/2 cup | 35 |
| Celery, raw | 2 stalks | 34 |
| Milk chocolate bar | 1 bar (1.02 oz) | 34 |
| Raspberries, black, raw | 1/2 cup | 33 |
| Orange, edible portion | 1 medium | 24 |
| Green beans, cooked | 1/2 cup | 23 |
| Chives, raw, chopped | 1 tablespoon | 19 |
| Leeks, raw | 1/2 medium | 15 |
| Blackberries, raw | 1/2 cup | 13 |
| Concord grapes | 1/2 cup | 13 |
| Blueberries, raw | 1/2 cup | 11 |
| Currants, red | 1/2 cup | 11 |
| Apricots, raw | 2 medium | 10 |
| Raspberries, red, raw | 1/2 cup | 10 |
| Broccoli, cooked | 1 large stalk | 6 |
| Cranberry juice | 1/2 cup (4 oz) | 6 |
Diuretics
Although it has been claimed that the diuretic effects of alcohol can result in dehydration, which is important for kidney stone sufferers to avoid, there are no conclusive data demonstrating any cause and effect regarding kidney stones. However, some have theorized that frequent and binge drinkers create situations that set up dehydration: alcohol consumption, hangovers, and poor sleep and stress habits. In this view, it is not the alcohol that creates a kidney stone but it is the alcohol drinker's associated behavior that sets it up.[42]
One of the recognized medical therapies for prevention of stones is thiazides, a class of drugs usually thought of as diuretics. These drugs prevent stones through an effect independent of their diuretic properties: they reduce urinary calcium excretion. Nonetheless, their diuretic property does not preclude their efficacy as stone preventive. Sodium restriction is necessary for clinical effect of thiazides, as sodium excess promotes calcium excretion. Though some have said that the effect probably fades after two years or so of therapy (tachyphylaxis), in fact it is only randomized controlled trials lasting 2 years or more that show the effect; there is really no good evidence from studies of calcium metabolism that the thiazide effect does not last indefinitely. Thiazides are the medical therapy of choice for most cases of hypercalciuria (excessive urinary calcium) but may not be suitable for all calcium stone formers; just those with high urinary calcium levels.
Allopurinol
Allopurinol (Zyloprim) is another drug with proven benefits in some calcium kidney stone formers. Allopurinol interferes with the liver's production of uric acid. Hyperuricosuria, too much uric acid in the urine, is a risk factor for calcium stones. Allopurinol reduces calcium stone formation in such patients. The drug is also used in patients with gout or hyperuricemia, but the latter is not the critical feature of uric acid stones.[43] Uric acid stones are more often caused by low urine pH. Even relatively high uric acid excretion will not be associated with uric acid stone formation if the urine pH is alkaline. Therefore prevention of uric acid stones relies on alkalinization of the urine with citrate.
Allopurinol is reserved for patients in whom alkalinization is difficult. For patients with increased uric acid levels and calcium stones, allopurinol is one of the few treatments that has been shown in double-blinded placebo controlled studies to actually reduce kidney stone recurrences. Dosage is adjusted to maintain a reduced urinary excretion of uric acid. Serum uric acid level at or below 6 mg/dL is often the goal of the drug's use in patients with gout or hyperuricemia.
Decreased protein diet
A high protein diet might be partially to blame. Protein from meat and other animal products is broken down into acids, including uric acid. The most available alkaline base to balance the acid from protein is calcium phosphate (hydroxyapatite) from the bones (buffering). The kidney filters the liberated calcium which may then form insoluble crystals (i.e., stones) in urine with available oxalate (partly from metabolic processes, partly from diet) or phosphate ions, depending on conditions. High protein intake is therefore associated with decreased bone density as well as stones. The acid load is associated with decreased urinary citrate excretion; citrate competes with oxalate for calcium and can thereby prevent stones.
In addition to increased fluid intake, one of the simplest fixes is to moderate animal protein consumption. However, despite epidemiologic data showing that greater protein intake is associated with more stones, randomized controlled trials of protein restriction have not shown reduced stone prevalence. In this regard, it is not just dietary calcium per se that may cause stone formation, but rather the leaching of bone calcium. Some diseases (e.g., distal renal tubular acidosis) which cause a chronically acidic state also decrease urinary citrate levels; since citrates are normally present as potent inhibitors of stone formation, these patients are prone to frequent stone formation.
Other modifications
Potassium citrate is also used in kidney stone prevention. This is available as both a tablet and liquid preparation. The medication increases urinary pH (makes it more alkaline), as well as increases the urinary citrate level, which helps reduce calcium oxalate crystal aggregation. Optimal 24 hour urine levels of citrate are thought to be over 320 mg/liter of urine or over 600 mg per day. There are urinary dipsticks available that allow patients to monitor and measure urinary pH so patients can optimize their urinary citrate level.
Though caffeine does acutely increase urinary calcium excretion, several independent epidemiologic studies have shown that coffee intake overall is protective against the formation of stones.[44]
Measurements of food oxalate content have been difficult and issues remain about the proportion of oxalate that is bio-available, versus a proportion that is not absorbed by the intestine. Oxalate-rich foods are usually restricted to some degree, particularly in patients with high urinary oxalate levels, but no randomized controlled trial of oxalate restriction has been performed to test that hypothesis.
Calgranulin
Crystallization of calcium oxalate (CaOx) appears to be reduced by molecules in the urine that retard the formation, growth, aggregation, and renal cell adherence of calcium oxalate. By purifying urine using salt precipitation, preparative isoelectric focusing, and sizing chromatography, some researchers have found that the molecule calgranulin is able to inhibit calcium oxalate crystal growth.[45] Calgranulin is a protein formed in the kidney. Given the large amounts of calcium oxalate in the urine, and considering its potency, calgranulin could become an important contribution to the normal urinary inhibition of crystal growth and aggregation. If so, it will be an important tool in the renal defense against kidney stones.
See also
- Nephrology
- Urinary retention
- Urology
- Retrograde pyelogram
- Cystinuria
- Intravenous pyelogram
- Kidney stone sufferers
References
- ↑ Chiras, Daniel D. (2007). Human Biology. Jones & Bartlett Publishers. ISBN 0763738433.
- ↑ Collins, C. Edward (2005). A Short Course in Medical Terminology. Lippincott Williams & Wilkins. ISBN 0781747678.
- ↑ 3.0 3.1 3.2 3.3 Weaver, S. H.; Jenkins, P. et al (2002). "Chapter 14: Renal and Urological Care". Illustrated Manual of Nursing Practice (3rd edition ed.). Lippincott Williams & Wilkins. ISBN 1582550824.
- ↑ Stamatelou, Kiriaki K.; Francis, Mildred E.; Jones, Camille A; Nyberg Jr., Leroy M.; Curhan, Gary C. (2003). "Time trends in reported prevalence of kidney stones in the United States: 1976–1994". Kidney International 63: 1817–1823. doi:.
- ↑ 5.0 5.1 5.2 5.3 5.4 Pietrow, Paul K.; Karellas, Michael E. (2006). "Medical Management of Common Urinary Calculi". American Family Physician 74 (1): 86–94. http://www.aafp.org/afp/20060701/86.html. Retrieved on 2008-05-20.
- ↑ Potts, Jeannette M. (2004). Essential Urology: A Guide to Clinical Practice. Humana Press. pp. 129. ISBN 158829109X.
- ↑ 7.0 7.1 7.2 7.3 Moe, Orson W. (2006). "Kidney stones: pathophysiology and medical management". The Lancet 367 (9507): 333–344. doi:.
- ↑ Tarkan, Laurie (2008-10-28). "A Rise in Kidney Stones Is Seen in U.S. Children".
- ↑ Eknoyan, Garabed (2004). "History of urolithiasis". Clinical Reviews in Bone and Mineral Metabolism 2 (3): 177–185. doi:.
- ↑ 10.0 10.1 Shah, J.; Whitfield, H. N. (2002). "Urolithiasis through the ages". BJU International 89 (8): 801–810. doi:.
- ↑ 11.0 11.1 Basler, Joseph; Ghobriel, Aldo; Talavera, Francisco; Resnick, Martin I.; Wolf, J. Stuart, Jr.; Leslie, Stephen W. (August 10, 2007). "Bladder Stones". WebMD. Retrieved on 2008-05-22.
- ↑ Auge, Brian K.; Preminger, Glenn M. (2002). "Update on shock wave lithotripsy technology". Current Opinion in Urology 12 (4): 287–290. doi:. http://www.ncbi.nlm.nih.gov/pubmed/12072648. Retrieved on 2008-05-22.
- ↑ Ciftçioglu, N.; Haddad, R. S.; Golden, D. C.; Morrison, D. R.; McKay D. S. (2005). "A potential cause for kidney stone formation during space flights: enhanced growth of nanobacteria in microgravity". Kidney International 67 (2): 483–91. doi:. http://www.ncbi.nlm.nih.gov/pubmed/15673296. Retrieved on 2008-05-22.
- ↑ Lloyd, S. E.; Pearce, S. H. S.; Fisher, S. E.; Steinmeyer, K.; Schwappach, B.; Scheinman, S. J.; Harding, B.; Bolino, A.; Devoto, M.; Goodyer, P.; Rigden, S. P. A.; Wrong, O.; Jentsch, T. J.; Craig, I. W.; Thakker, R. V. (1996). "A common molecular basis for three inherited kidney stone diseases". Nature 379: 445–449. doi:.
- ↑ Ginalski, J. M.; Portmann, L.; Jaeger, P. (1991). "Does medullary sponge kidney cause nephrolithiasis?". American Journal of Roentgenology 156 (4): 872–3. PMID 2115256. http://www.ajronline.org/cgi/content/abstract/155/2/299. Retrieved on 2008-03-12.
- ↑ ccfa.org
- ↑ National Research Council (2002). Fluoride in Drinking Water: A Scientific Review of EPA's Standard. New York: National Academies Press. ISBN 030910128X.
- ↑ Smith, Donald Ridgeway; Tanagho, Emil A.; McAninch, Jack W. (2003). Smith's General Urology. McGraw-Hill Professional. ISBN 0071396489.
- ↑ Goodwin JS, Tangum MR (November 1998). "Battling quackery: attitudes about micronutrient supplements in American academic medicine". Arch. Intern. Med. 158 (20): 2187–91. PMID 9818798. http://archinte.ama-assn.org/cgi/pmidlookup?view=long&pmid=9818798.
- ↑ Massey LK, Liebman M, Kynast-Gales SA (2005). "Ascorbate increases human oxaluria and kidney stone risk". J. Nutr. 135 (7): 1673–7. PMID 15987848. http://jn.nutrition.org/cgi/reprint/135/7/1673.pdf.
- ↑ Naidu KA (2003). "Vitamin C in human health and disease is still a mystery? An overview.". J. Nutr. 2 (7): 7. doi:. PMID 14498993. http://www.nutritionj.com/content/pdf/1475-2891-2-7.pdf.
- ↑ 22.0 22.1 22.2 22.3 Parmar, Malvinder S. (2004). "Kidney stones". British Medical Journal 328 (7453): 1420–1424. doi:. PMID 15191979.
- ↑ Halabe A, Sperling O (1994). "Uric acid nephrolithiasis". Mineral and electrolyte metabolism 20 (6): 424–31. PMID 7783706.
- ↑ www.utdol.com
- ↑ Mayo Clinic (2008). "Kidney Stone Channel". U.S. News & World Report. Retrieved on 2008-04-23.
- ↑ Kumar, Vinay; Fausto, Nelson; Fausto, Nelso; Robbins, Stanley L.; Abbas, Abul K.; Cotran, Ramzi S. (2005). Robbins and Cotran Pathologic Basis of Disease (7th ed.). Philadelphia, Pa.: Elsevier Saunders. pp. 1012. ISBN 0-7216-0187-1.
- ↑ Goljan, Edward F. (2007). Rapid Review Pathology (2nd ed.). Mosby. pp. 397. ISBN 0-323-04414-X.
- ↑ Anonymous (October 2007). "How are kidney stones treated?". Kidney Stones in Adults. National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved on 2008-05-20.
- ↑ Anonymous (2008). "Kidney Stones". Cape Fear Valley Medical Center. Retrieved on 2008-05-20.
- ↑ Reilly, Robert F. (2005). Nephrology in 30 Days. UNC Press. pp. 195. ISBN 1882886208.
- ↑ Chang, J. M.; Hwang, S. J.; Kuo, H. T.; Tsai, J. C.; Guh, J. Y.; Chen, H. C.; Tsai, J. H.; Lai, Y. H. (2000). "Fatal outcome after ingestion of star fruit (Averrhoa carambola) in uremic patients.". American Journal of Kidney Diseases 35 (2): 189–93. doi:. http://www.ncbi.nlm.nih.gov/pubmed/10676715. Retrieved on 2008-05-12.
- ↑ Krambeck, A.; Gettman, M.; Rohlinger, A.; Lohse, C.; Patterson, D.; Segura, J. (2006). "Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup". The Journal of Urology 175 (5): 1742–7. doi:. PMID 16600747.
- ↑ Edelson, Ed (April 12, 2006). "Kidney Stone Shock Wave Treatment Boosts Diabetes, Hypertension Risk - Study suggests link, but doctors say it's too early to abandon this therapy", HealthFinder, National Health Information Center. Retrieved on 2008-05-03.
- ↑ Goldfarb, David S.; Coe, Fredric L. (November 15, 1999). "Prevention of recurrent nephrolithiasis". American Family Physician 60 (8): 2269–76. PMID 10593318. http://www.aafp.org/afp/991115ap/2269.html.
- ↑ Hassell, Beverly (2001-08-28). "Too much soy could lead to kidney stones". EurekAlert. Retrieved on 2008-06-28.
- ↑ Kelera,, T.; Jansen, B.; Hesse, A. (2002). "Effect of blackcurrant-, cranberry- and plum juice consumption on risk factors associated with kidney stone formation". European Journal of Clinical Nutrition 56 (10): 1020–1023. doi:. http://www.nature.com/ejcn/journal/v56/n10/abs/1601442a.html. Retrieved on 2008-05-12.
- ↑ Odvina, Clarita V. (2006). "Comparative Value of Orange Juice versus Lemonade in Reducing Stone-Forming Risk". Clinical Journal of the American Society of Nephrology 1 (6): 1269–74. doi:. PMID 17699358. http://cjasn.asnjournals.org/cgi/content/abstract/1/6/1269. Retrieved on 2008-05-12.
- ↑ O'Connor, Anahad (January 22, 2008). "The Claim: Too Much Cola Can Cause Kidney Problems", The New York Times. Retrieved on 2008-04-23.
- ↑ Saldana, Tina M.; Basso, Olga; Darden, Rebecca; Sandler, Dale P. (2007). "Carbonated Beverages and Chronic Kidney Disease". Epidemiology 18 (4): 501–506. doi:. http://www.epidem.com/pt/re/epidemiology/abstract.00001648-200707000-00017.htm. Retrieved on 2008-05-12.
- ↑ Taylor, Eric N. coauthors=Stampfer, Meir J.; Curhan, Gary C. (2004). "Dietary Factors and the Risk of Incident Kidney Stones in Men: New Insights after 14 Years of Follow-up". Journal of the American Society of Nephrology 15 (6): 3225–3232. doi:. PMID 15579526.
- ↑ Resnick, Martin I.; Pak, Charles Y. C. (1990). Urolithiasis, A Medical and Surgical Reference. W.B. Saunders Company. pp. 158. ISBN 0721624391.
- ↑ Rodman, John S.; Seidman, Cynthia (1996). No More Kidney Stones. Wiley. ISBN 0471125873.
- ↑ Cameron, J. S.; Simmonds, H. A. (1987). "Use and abuse of allopurinol.". British Medical Journal 295 (6594): 1504–1505. PMID 3607420. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1246665. Retrieved on 2008-05-23.
- ↑ Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ (1996, February 1). "Prospective Study of Beverage Use and the Risk of Kidney Stones". Am Jour Epidemiology 143 (3): 240–247. PMID 8561157. http://aje.oxfordjournals.org/cgi/content/abstract/143/3/240.
- ↑ Pillay, Sokalingum N.; Asplin, John R.; Coe, Fredric L. (1998). "Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization". American Journal of Physiology - Renal Physiology 275: F255–F261. PMID 9691016. http://ajprenal.physiology.org/cgi/content/abstract/275/2/F255. Retrieved on 2008-04-23.
External links
- Pictures of kidney stones, showing their crystalline shape
- National Kidney and Urologic Diseases Information Clearinghouse
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||