Kevlar
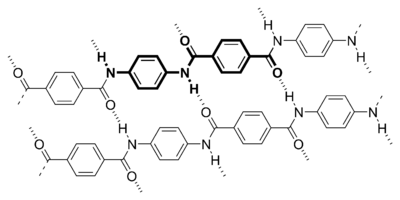
Kevlar is the registered trademark for a light, strong para-aramid synthetic fiber, related to other aramids such as Nomex and Technora.
Developed at DuPont in 1965 by Stephanie Kwolek [1] [2] it was first commercially used in the early 1970s as a replacement for steel in racing tires. Typically it is spun into ropes or fabric sheets that can be used as such or as an ingredient in composite material components.
Currently, Kevlar has many applications, ranging from bicycle tires and racing sails to body armor because of its high strength-to-weight ratio—famously: "...5 times stronger than steel on an equal weight basis..."[2]
A similar fiber called Twaron with roughly the same chemical structure was introduced by Akzo in 1978, and now manufactured by Teijin.
Contents |
Properties
When Kevlar is spun, the resulting fiber has great tensile strength (ca. 3 620 MPa), and a relative density of 1.44. When used as a woven material, it is suitable for mooring lines and other underwater applications.
There are three grades of Kevlar: (i) Kevlar, (ii) Kevlar 29, and (iii) Kevlar 49. Typically, Kevlar is used as reinforcement in tires and rubber mechanical goods. Kevlar 29's industrial applications are as cables, in asbestos replacement, brake linings, and body armor. Kevlar 49 has the greatest tensile strength of all the aramids, and is used in plastic reinforcement for boat hulls, airplanes, and bicycles. The ultraviolet light component of sunlight degrades and decomposes Kevlar, a problem known as UV degradation, and so it is rarely used outdoors without protection against sunlight.
Production
Kevlar is synthesised in solution from the monomers 1,4-phenylene-diamine (para-phenylenediamine) and terephthaloyl chloride in a condensation reaction yielding hydrochloric acid as a byproduct. The result has liquid-crystalline behaviour, and mechanical drawing orients the polymer chains in the fiber's direction. Hexamethylphosphoramide (HMPA) was the polymerization solvent first used, but toxicology tests demonstrated it provoked tumors in the noses of rats, so DuPont replaced it by a N-methyl-pyrrolidone and calcium chloride as the solvent. As this process was patented by Akzo (see above) in the production of Twaron, a patent war ensued.
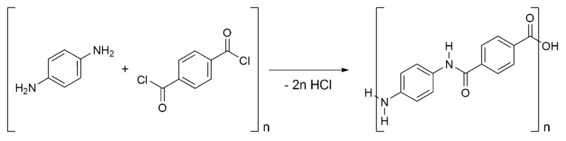
Kevlar (poly paraphenylene terephthalamide) production is expensive because of the difficulties arising from using concentrated sulfuric acid, needed to keep the water-insoluble polymer in solution during its synthesis and spinning.
Chemical properties
Fibers of Kevlar consist of long molecular chains produced from PPTA (poly-paraphenylene terephthalamide). There are many inter-chain bonds making the material extremely strong. Kevlar derives part of its high strength from inter-molecular hydrogen bonds formed between the carbonyl groups and protons on neighboring polymer chains and the partial pi stacking of the benzenoid aromatic stacking interactions between stacked strands. These interactions have a greater influence on Kevlar than the van der Waals interactions and chain length that typically influence the properties of other synthetic polymers and fibers such as Dyneema. The presence of salts and certain other impurities, especially calcium, could interfere with the strand interactions and caution is used to avoid inclusion in its production. Kevlar's structure consists of relatively rigid molecules which tend to form mostly planar sheet-like structures rather like silk protein.
Thermal properties
For a polymer, Kevlar has very good resistance to high temperatures, and maintains its strength and resilience down to cryogenic temperatures (-196°C); indeed, it is slightly stronger at low temperatures.
At higher temperatures the tensile strength is immediately reduced by about 10-20%, and after some hours the strength progressively reduces further. For example at 160°C about 10% reduction in strength occurs after 500 hours. At 260°C 50% strength reduction occurs after 70 hours.[3]
At 450°C Kevlar sublimates.
Applications
Armor
Kevlar is well-known as a component of some bulletproof vests and bulletproof facemasks. The PASGT helmet and vest used by United States military forces since the early 1980s both have Kevlar as a key component, as do their replacements. Other military uses include bulletproof facemasks used by sentries. Civilian applications include Kevlar reinforced clothing for motorcycle riders to protect against abrasion injuries and also Emergency Service's protection gear if it involves high heat (e.g., tackling a fire), and Kevlar body armor such as vests for police officers, security, and SWAT.
Rope and cable
The fiber is used in woven rope and in cable, where the fibers are kept parallel within a polyethylene sleeve. Known as "Parafil", the cables have been used in small suspension bridges such as the bridge at Aberfeldy in Scotland. They have also been used to stabilise cracking concrete cooling towers by circumferential application followed by tensioning to close the cracks.
Sports equipment

It is used as an inner lining for some bicycle tires to prevent punctures, and due to its excellent heat resistance, is used for fire poi wicks. It is used for motorcycle safety clothing, especially in the areas featuring padding such as shoulders and elbows. It was also used as speed control patches for certain Soap Shoes models. In Kyudo or Japanese archery, it may be used as an alternative to more expensive hemp for bow strings. It is one of the main materials used for paraglider suspension lines.
Audio equipment
It has also been found to have useful acoustic properties for loudspeaker cones, specifically for bass and midrange drive units[4].
Electricity generation
Kevlar was used by scientists at Georgia Institute of Technology as a base textile for an experiment in electricity-producing clothing. This was done by weaving zinc oxide nanowires into the fabric. If successful, the new fabric would generate about 80 milliwatts per square meter.[5]
Drumheads
Kevlar is sometimes used as a material in high tension drum heads usually used on marching snare drums. It supposedly gives a higher pitched sound and allows for an extremely high amount of tension. There is usually some sort of resin poured onto the kevlar to prevent the air inside the drum from escaping through the head. This is one of the primary types of marching snare drum heads. Remo's falam slam patch is made with kevlar and is stuck to bass drum heads where the beater strikes to strengthen the head so it doesn't wear down as quickly.
Woodwind reeds
Kevlar is used in the woodwind reeds of Fibracell. The material of these reeds is a composite of aerospace materials designed to duplicate the way nature constructs cane reed. Very stiff but sound absorbing Kevlar fibers are suspended in a lightweight resin formulation.
Fiber Optic Cable
Kevlar is widely used as a protective outer sheath for fiber optic cable, as its strength protects the cable from damage and kinking.
Building construction
A retractable roof of over 60,000 square feet (5,575 square metres) of Kevlar was a key part of the design of Montreal's Olympic stadium for the 1976 Summer Olympics. It was spectacularly unsuccessful, as it was completed ten years late and replaced just ten years later in May 1998 after a series of problems.[6][7]
Brakes
The chopped fiber has been used as a replacement for asbestos in brake pads. Dust produced from asbestos brakes is toxic, while aramids are a benign substitute.
Expansion joints and hoses
Kevlar can be found as a reinforcing layer in rubber bellows expansion joints and rubber hoses, for use in high temperature applications, and for its high strength. It is also found as a braid layer used on the outside of hose assemblies, to add protection against sharp objects.
Composite materials
Aramid fibers are widely used for reinforcing composite materials, often in combination with carbon fiber and glass fiber. The matrix for high performance composites is usually epoxy resin. Typical applications include monocoque bodies for F1 racing cars, helicopter rotor blades, tennis, table tennis, badminton and squash rackets, kayaks, cricket bats, and field hockey, ice hockey and lacrosse sticks. [8] [9] [10] [11]
See also
- Aramid
- Bulletproof vest
- DuPont (Company that invented and manufactures Kevlar)
- Interceptor body armor
- Nomex
- Vectran
- Personnel Armor System for Ground Troops
- Spider silk
- Twaron
- Ultra high molecular weight polyethylene
- UV degradation
- Soap Shoes
- Fire dancing
References
- ↑ MIT - Stephanie Kwolek bio
- ↑ 2.0 2.1 "What is Kevlar". DuPont. Retrieved on 2007-03-28.
- ↑ KEVLAR Technical Guide
- ↑ Audio speaker use
- ↑ Scientific American: Fabric Produces Electricity As You Wear It
- ↑ Roof of the Montreal Olympic Stadium in the Structurae database
- ↑ Clem's Baseball ~ Olympic Stadium
- ↑ Kadolph, Sara J. Anna L. Langford. Textiles, Ninth Edition. Pearson Education, Inc 2002. Upper Saddle River, NJ
- ↑ D. Tanner, J. A. Fitzgerald, B. R. Phillips (1989). "The Kevlar Story - an Advanced Materials Case Study". Angewandte Chemie International Edition in English 28 (5): 649–654. doi:.
- ↑ E. E. Magat (1980). "Fibers from Extended Chain Aromatic Polyamides, New Fibers and Their Composites". Philosophical Transactions of the Royal Society of London Series A 294 (1411): 463–472. http://links.jstor.org/sici?sici=0080-4614%2819800121%29294%3A1411%3C463%3AFFECAP%3E2.0.CO%3B2-P.
- ↑ Ronald V. Joven. Manufacturing Kevlar panels by thermo-curing process. Los Andes University, 2007. Bogotá, Colombia.
External links
- Kevlar Home Page
- Aramids
- Kevlar - Design Dictionary. Illustrated article about Kevlar
- Matweb material properties of Kevlar
- U.S. Patent 5,565,264
- Kevlar
- Synthesis of Kevlar
- Aberfeldy Footbridge over the River Tay
|
||||||||||||||
|
||||||||||||||||||
