Ketone
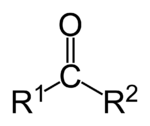
A ketone (pronounced as key tone) is either the functional group characterized by a carbonyl group (O=C) linked to two other carbon atoms or a chemical compound that contains a carbonyl group. A ketone can be generally represented by the chemical formula:
A carbonyl carbon bonded to two carbon atoms distinguishes ketones from carboxylic acids, aldehydes, esters, amides, and other oxygen-containing compounds. The double-bond of the carbonyl group distinguishes ketones from alcohols and ethers. The simplest ketone is acetone, CH3-CO-CH3 (systematically named propanone[1]).
The carbon atom adjacent to a carbonyl group is called the α-carbon. Hydrogens attached to this carbon are called α-hydrogens. In the presence of an acid catalyst the ketone is subjected to so-called keto-enol tautomerism. The reaction with a strong base gives the corresponding enolate. A diketone is a compound containing two ketone groups.
Contents |
Nomenclature
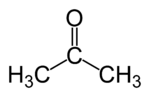
In general, ketones are named using IUPAC nomenclature by changing the suffix -e of the parent alkane to -one. For common ketones, some traditional names such as acetone and benzophenone predominate, and these are considered retained IUPAC names [2], although some introductory chemistry texts use names such as propanone.
Oxo is the formal IUPAC nomenclature for a ketone functional group. However, other prefixes are also used by various books and journals. For some common chemicals (mainly in biochemistry), keto or oxo is the term used to describe the ketone (also known as alkanone) functional group. Oxo also refers to a single oxygen atom coordinated to a transition metal (a metal oxo).
Physical properties
A carbonyl group is polar. This makes ketones polar compounds. The carbonyl groups interact with water by hydrogen bonding, and ketones are soluble in water. It is a hydrogen-bond acceptor, but not a hydrogen-bond donor, and cannot hydrogen-bond to itself. This makes ketones more volatile than alcohols and carboxylic acids of similar molecular weight.
Acidity
The α-hydrogen of a ketone is far more acidic (pKa ≈ 20) than the hydrogen of a regular alkane (pKa ≈ 50). This is due to resonance stabilization of the enolate ion that is formed through dissociation. The relative acidity of the α-hydrogen is important in the enolization reactions of ketones and other carbonyl compounds.
Spectroscopic properties
Spectroscopy is an important means for identifying ketones.Ketones and aldehydes will display a significant peak in infrared spectroscopy, at around 1700 cm−1 (slightly higher or lower, depending on the chemical environment).
While 1H NMR is generally not useful for revealing the presence of a ketone, 13C NMR spectra exhibit (typically relatively weak) signals somewhat downfield of 200 ppm depending on structure. Since aldehydes resonate at similar chemical shifts, multiple different NMR experiments are required to definitively distinguish aldehydes and ketones spectrometrically.
Synthesis
Many methods exist for the preparation of ketones in the laboratory or on industrial scale, including:
- Ketones can be created by oxidation of secondary alcohols, as in the oxidation of propan-2-ol to acetone: H3C-CH(OH)-CH3 → H3C-CO-CH3.
Classically, such reactions required a strong oxidant such as potassium permanganate or a Cr(VI) compound. In modern organic synthesis, much milder conditions such as use of the Dess-Martin periodinane or the Moffatt-Swern oxidation are commonly employed.
- Ketones can be prepared by Gem halide hydrolysis.
- Alkynes can be turned into enols through a hydration reaction in the presence of an acid and HgSO4, and subsequent enol-keto tautomerization gives a ketone. This always produces a ketone, even with a terminal alkyne, and disiamylborane is needed to get an aldehyde from an alkyne
- Aromatic ketones can be prepared in the Friedel-Crafts reaction, the related Houben-Hoesch reaction and the Fries rearrangement.
- Ozonolysis, and related dihydroxylation/oxidative sequences, cleave alkenes to give aldehydes and/or ketones, depending on alkene substitution pattern.
- In the Kornblum–DeLaMare rearrangement ketones are prepared from peroxides and base.
- In the Ruzicka cyclization, cyclic ketones are prepared from dicarboxylic acids.
- In the Nef reaction, ketones form by hydrolysis of salts of secondary nitro compounds.
- In the Fukuyama coupling, ketones form from a thioester and an organozinc compound.
- Ketones can be prepared by the reaction of an acid chloride with organocadmium compounds or organocopper compounds.
- The Dakin-West reaction provides an efficient method for preparation of certain methyl ketones from carboxylic acids.
Reactions
Ketones engage in many organic reactions:
- Nucleophilic addition. The reaction of a ketone with a nucleophile gives a tetrahedral carbonyl addition compound.
- the reaction with the anion of a terminal alkyne gives a hydroxyalkyne
- the reaction with ammonia or a primary amine gives an imine + water
- the reaction with secondary amine gives an enamine + water
- the reaction with a Grignard reagent gives a magnesium alkoxide and after aqueous workup a tertiary alcohol
- the reaction with an organolithium reagent also gives a tertiary alcohol
- the reaction with an alcohol, an acid or base gives a hemiketal + water and further reaction with an alcohol gives the ketal + water. This is a carbonyl-protecting reaction.
- reaction of RCOR' with sodium amide results in cleavage with formation of the amide RCONH2 and the alkane R'H, a reaction called the Haller-Bauer reaction (1909) [3]
- Electrophilic addition, reaction with an electrophile gives a resonance stabilized cation.
- the reaction with phosphonium ylides in the Wittig reaction gives alkenes
- reaction with water gives geminal diols
- reaction with thiols gives a thioacetal
- reaction with hydrazine or derivatives of hydrazine gives hydrazones
- reaction with a metal hydride gives a metal alkoxide salt and then with water an alcohol
- reaction of an enol with halogens to form α-haloketone
- a reaction at an α-carbon is the reaction of a ketone with heavy water to give a deuterated ketone-d.
- fragmentation in photochemical Norrish reaction
- reaction with halogens and base of methyl ketones in the Haloform reaction
- reaction of 1,4-aminodiketones to oxazoles by dehydration in the Robinson-Gabriel synthesis
- reaction of aryl alkyl ketones with sulfur and an amine to amides in the Willgerodt reaction
Biochemistry
Acetone, acetoacetate and beta-hydroxybutyrate are ketones (or ketone bodies) generated from carbohydrates, fatty acids and amino acids in humans and most vertebrates. Ketones are elevated in blood after fasting including a night of sleep, and in both blood and urine in starvation, hypoglycemia due to causes other than hyperinsulinism, various inborn errors of metabolism, and ketoacidosis (usually due to diabetes mellitus). Although ketoacidosis is characteristic of decompensated or untreated type 1 diabetes, ketosis or even ketoacidosis can occur in type 2 diabetes in some circumstances as well. Acetoacetate and beta-hydroxybutyrate are an important fuel for many tissues, especially during fasting and starvation. The brain, in particular, relies heavily on ketone bodies as a substrate for lipid synthesis and for energy during times of reduced food intake. At the NIH, Dr. Richard Veech refers to ketones as "magic" in their ability to increase metabolic efficiency, while decreasing production of free radicals, the damaging byproducts of normal metabolism. His work has shown that ketone bodies may treat neurological diseases such as Alzheimer's and Parkinson's disease,[4] and the heart and brain operate 25% more efficiently using ketones as a source of energy.[5] Research has also shown ketones play a role in reducing epileptic seizures with the so-called high-fat, near-zero carbohydrate Ketogenic Diet. [1]
Ketones are mainly caused by high blood sugars.
Applications
Ketones are often used in perfumes and paints to stabilize the other ingredients so that they don't degrade as quickly over time. Other uses are as solvents and intermediates in chemical industry. Examples of ketones are acetone, acetophenone, and methyl ethyl ketone.
See also
- List of publications in organic chemistry
- Ketosis
- Ketone bodies (Medical term referring to all or either of the three ketones occurring in the body).
References
- ↑ The position of the carbonyl group is usually denoted by a number; in propanone there can only be one position. While propanone or propan-2-one is how the molecule should be named according to systematic nomenclature, the name "acetone" is retained in official IUPAC nomenclature
- ↑ List of retained IUPAC names retained IUPAC names Link
- ↑ Haller-Bauer Reaction
- ↑ Y. Kashiwaya, T. Takeshima, N. Mori, K. Nakashima, K. Clarke and R. L. Veech (2000). "D-beta -Hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease". PNAS 97 (10): 5440–5444. doi:. PMID 10805800.
- ↑ Y. Kashiwaya, K. Sato, N. Tsuchiya, S. Thomas, D. A. Fell, R. L. Veech and J. V. Passonneau (1994). "Control of glucose utilization in working perfused rat heart". J. Biol. Chem. 269 (41): 25502–25514. PMID 7929251. http://www.jbc.org/cgi/content/abstract/269/41/25502.
|
|||||