Isomer
- This article is about the chemical concept. For "isomerism" of atomic nuclei, see nuclear isomer.
In chemistry, isomers (Greek isos = "equal", méros = "part") are compounds with the same molecular formula but different structural formulae.[1] Isomers do not necessarily share similar properties unless they also have the same functional groups. This should not be confused with a nuclear isomer, which involves a nucleus at different states of excitement. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical isomers, et cetera (see graph below).
Contents |
Isomerism
A simple example of isomerism is given by propanol: it has the formula C3H8O (or C3H7OH) and occurs as two isomers: propan-1-ol (n-propyl alcohol; I) and propan-2-ol (isopropyl alcohol; II)
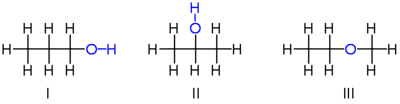
Note that the position of the oxygen atom differs between the two: it is attached to an end carbon in the first isomer, and to the center carbon in the second.
There is, however, another isomer of C3H8O which has significantly different properties: methoxyethane (methyl-ethyl-ether; III). Unlike the isomers of propanol, methoxyethane has an oxygen atom that is connected to two carbons rather than to one carbon and one hydrogen. This makes it an ether, not an alcohol, as it lacks a hydroxyl group, and has chemical properties more similar to other ethers than to either of the above alcohol isomers.
Examples of isomers having different medical properties can be easily found. For example, in the placement of methyl groups. In substituted xanthines, Theobromine, found in chocolate, is a vasodilator with some effects in common with caffeine, but if one of the two methyl groups is moved to a different position on the two-ring core, the isomer is theophylline, which has a variety of effects, including bronchodilation and anti-inflammatory action. Another example of this occurs in the phenethylamine-based stimulant drugs. Phentermine is a non-chiral compound with a weaker effect than amphetamine. It is used as an appetite reducing medication and has mild or no stimulant properties. However, a different rearrangement of the same atoms gives dextromethamphetamine, which is more potent than amphetamine; it is a very strong stimulant.
Allene and propyne are examples of isomers containing different bond types. Allene contains two double bonds, while propyne contains one triple bond.
Classification

There are two main forms of isomerism: structural isomerism and stereoisomerism.
In structural isomers, the atoms and functional groups are joined together in different ways, as in the example of propyl alcohol above. This group includes chain isomerism whereby hydrocarbon chains have variable amounts of branching; position isomerism which deals with the position of a functional group on a chain; and functional group isomerism in which one functional group is split up into different ones.
In stereoisomers the bond structure is the same, but the geometrical positioning of atoms and functional groups in space differs. This class includes enantiomers where different isomers are non-superimposable mirror-images of each other, and diastereomers when they are not. Diastereomerism is again subdivided into conformational isomerism (conformers) when isomers can interconvert by chemical bond rotations and cis-trans isomerism when this is not possible. Note that although conformers can be referred to as having a diastereomeric relationship, the isomers over all are not diastereomers, since bonds in conformers can be rotated to make them mirror images.
In skeletal isomers the main carbon chain is different between the two isomers. This type of isomerism is most identifiable in secondary and tertiary alcohol isomers.
Tautomers are structural isomers of the same chemical substance that spontaneously interconvert with each other, even when pure. They have different chemical properties, and consequently, distinct reactions characteristic to each form are observed. If the interconversion reaction is fast enough, tautomers cannot be isolated from each other. An example is when they differ by the position of a proton, such as in keto/enol tautomerism, where the proton is alternately on the carbon or oxygen.
In food chemistry, medicinal chemistry and biochemistry, cis-trans isomerism is always considered. In medicinal chemistry and biochemistry, enantiomers are of particular interest since most changes in these types of isomers are now known to be meaningful in living organisms. Pharmaceutical and academic researchers have found chromatographical methods to reliably separate these from each other. On an industrial scale, however, these methods are rather costly and are mostly used to filter out the potentially harmful or biologically inactive enantiomer.
While structural isomers typically have different chemical properties, stereoisomers behave identically in most chemical reactions, except in their reaction with other stereoisomers. Enzymes however can distinguish between different enantiomers of a compound, and organisms often prefer one isomer over the other. Some stereoisomers also differ in the way they rotate polarized light.
Other types of isomerism exist outside this scope. Topological isomers called topoisomers are generally large molecules that wind about and form different shaped knots or loops. Molecules with topoisomers include catenanes and DNA. Topoisomerase enzymes can knot DNA and thus change its topology. There are also isotopomers or isotopic isomers that have the same numbers of each type of isotopic substitution but in chemically different positions. In nuclear physics, nuclear isomers are excited states of atomic nuclei. Spin isomers have differing distributions of spin among their constituent atoms.
History
Isomerism was first noticed in 1827, when Friedrich Woehler prepared cyanic acid and noted that although its elemental composition was identical to fulminic acid (prepared by Justus von Liebig the previous year), its properties were quite different. This finding challenged the prevailing chemical understanding of the time, which held that chemical compounds could be different only when they had different elemental compositions. After additional discoveries of the same sort were made, such as Woehler's 1828 discovery that urea had the same atomic composition as the chemically distinct ammonium cyanate, Jöns Jakob Berzelius introduced the term isomerism to describe the phenomenon.[2]
In 1849, Louis Pasteur separated tiny crystals of tartaric acid into their two mirror-image forms. The individual molecules of each were the left and right optical stereoisomers, solutions of which rotate the plane of polarized light in opposite directions.
See also
- Structural isomerism
- Cis-trans isomerism
- Isotopomers
- Isotopologue
- Chirality (chemistry)
References
- ↑ Neuss, Geoffrey; Chemistry Course Companion for the IB Diploma Programme; Oxford University Press; First Published 2007
- ↑ Esteban, Soledad. (2008). "Liebig–Wöhler Controversy and the Concept of Isomerism". J. Chem. Educ. 85: 1201. http://jchemed.chem.wisc.edu/Journal/Issues/2008/Sep/abs1201.html.